Multifaceted therapeutic targeting of ovarian peritoneal carcinomatosis through virus-induced immunomodulation
- PMID: 23299799
- PMCID: PMC3594021
- DOI: 10.1038/mt.2012.228
Multifaceted therapeutic targeting of ovarian peritoneal carcinomatosis through virus-induced immunomodulation
Abstract
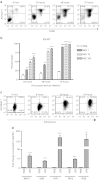
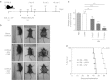
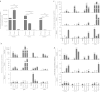
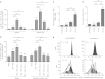
Immunosuppression associated with ovarian cancer (OC) and resultant peritoneal carcinomatosis (PC) hampers the efficacy of many promising treatment options, including immunotherapies. It is hypothesized that oncolytic virus-based therapies can simultaneously kill OC and mitigate immunosuppression. Currently, reovirus-based anticancer therapy is undergoing phase I/II clinical trials for the treatment of OC. Hence, this study was focused on characterizing the effects of reovirus therapy on OC and associated immune microenvironment. Our data shows that reovirus efficiently killed OC cells and induced higher expression of the molecules involved in antigen presentation including major histocompatibility complex (MHC) class I, β2-microglobulin (β2M), TAP-1, and TAP-2. In addition, in the presence of reovirus, dendritic cells (DCs) overcame the OC-mediated phenotypic suppression and successfully stimulated tumor-specific CD8+ T cells. In animal studies, reovirus targeted local and distal OC, alleviated the severity of PC and significantly prolonged survival. These therapeutic effects were accompanied by decreased frequency of suppressive cells, e.g., Gr1.1+, CD11b+ myeloid derived suppressor cells (MDSCs), and CD4+, CD25+, FOXP3+ Tregs, tumor-infiltration of CD3+ cells and higher expression of Th1 cytokines. Finally, reovirus therapy during early stages of OC also resulted in the postponement of PC development. This report elucidates timely information on a therapeutic approach that can target OC through clinically desired multifaceted mechanisms to better the outcomes.
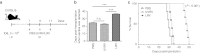
Figures

Similar articles
-
Role of Myeloid Cells in Oncolytic Reovirus-Based Cancer Therapy.Viruses. 2021 Apr 10;13(4):654. doi: 10.3390/v13040654. Viruses. 2021. PMID: 33920168 Free PMC article. Review.
-
Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis.J Immunother Cancer. 2021 Mar;9(3):e002096. doi: 10.1136/jitc-2020-002096. J Immunother Cancer. 2021. PMID: 33722907 Free PMC article.
-
Therapy-Induced MHC I Ligands Shape Neo-Antitumor CD8 T Cell Responses during Oncolytic Virus-Based Cancer Immunotherapy.J Proteome Res. 2019 Jun 7;18(6):2666-2675. doi: 10.1021/acs.jproteome.9b00173. Epub 2019 May 29. J Proteome Res. 2019. PMID: 31095916 Free PMC article.
-
Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms.Br J Cancer. 2014 Jan 7;110(1):83-93. doi: 10.1038/bjc.2013.695. Epub 2013 Nov 26. Br J Cancer. 2014. PMID: 24281006 Free PMC article.
-
Programmed death-1 pathway blockade produces a synergistic antitumor effect: combined application in ovarian cancer.J Gynecol Oncol. 2017 Sep;28(5):e64. doi: 10.3802/jgo.2017.28.e64. Epub 2017 Jun 5. J Gynecol Oncol. 2017. PMID: 28657225 Free PMC article. Review.
Cited by
-
Role of Myeloid Cells in Oncolytic Reovirus-Based Cancer Therapy.Viruses. 2021 Apr 10;13(4):654. doi: 10.3390/v13040654. Viruses. 2021. PMID: 33920168 Free PMC article. Review.
-
The golden key to open mystery boxes of SMARCA4-deficient undifferentiated thoracic tumor: focusing immunotherapy, tumor microenvironment and epigenetic regulation.Cancer Gene Ther. 2024 May;31(5):687-697. doi: 10.1038/s41417-024-00732-4. Epub 2024 Feb 13. Cancer Gene Ther. 2024. PMID: 38347129 Free PMC article. Review.
-
Phase 1b study of intraperitoneal ipilimumab and nivolumab in patients with recurrent gynecologic malignancies with peritoneal carcinomatosis.Med. 2024 Apr 12;5(4):311-320.e3. doi: 10.1016/j.medj.2024.02.003. Epub 2024 Mar 11. Med. 2024. PMID: 38471508 Clinical Trial.
-
Improving antitumor efficacy via combinatorial regimens of oncolytic virotherapy.Mol Cancer. 2020 Nov 10;19(1):158. doi: 10.1186/s12943-020-01275-6. Mol Cancer. 2020. PMID: 33172438 Free PMC article. Review.
-
Therapeutic Efficacy of Oncolytic Viruses in Fighting Cancer: Recent Advances and Perspective.Oxid Med Cell Longev. 2022 Jul 22;2022:3142306. doi: 10.1155/2022/3142306. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35910836 Free PMC article. Review.
References
-
- Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–116. - PubMed
-
- Barnett B, Kryczek I, Cheng P, Zou W., and, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. - PubMed
-
- Thibodeaux SR., and, Curiel TJ. Immune therapy for ovarian cancer: promise and pitfalls. Int Rev Immunol. 2011;30:102–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

