Microbial translocation in the pathogenesis of HIV infection and AIDS
- PMID: 23297256
- PMCID: PMC3553668
- DOI: 10.1128/CMR.00050-12
Microbial translocation in the pathogenesis of HIV infection and AIDS
Abstract
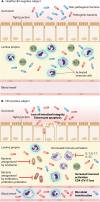
In pathogenic simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV) infections, the translocation of microbial products from the gastrointestinal (GI) tract to portal and systemic circulation has been proposed as a major driver of the chronic immune activation that is associated with disease progression. Consistently, microbial translocation is not present in nonpathogenic SIV infections of natural host species. In vivo studies demonstrated that HIV/SIV-associated microbial translocation results from a series of immunopathological events occurring at the GI mucosa: (i) early and severe mucosal CD4(+) depletion, (ii) mucosal immune hyperactivation/persistent inflammation; (iii) damage to the integrity of the intestinal epithelium with enterocyte apoptosis and tight junction disruption; and (iv) subverted the gut microbiome, with a predominance of opportunistic bacteria. Direct in situ evidence of microbial translocation has been provided for SIV-infected rhesus macaques showing translocated microbial products in the intestinal lamina propria and distant sites. While the mechanisms by which microbial translocation causes immune activation remain controversial, a key pathogenic event appears to be innate immunity activation via Toll-like receptors and other pathogen recognition receptors. Accumulating clinical observations suggest that microbial translocation might affect HIV disease progression, response to therapy, and non-AIDS comorbidities. Given its detrimental effect on overall immunity, several interventions to prevent/block microbial translocation are currently under investigation as novel therapeutic agents for HIV/AIDS.
Figures




Similar articles
-
Walk on the wild side: SIV infection in African non-human primate hosts-from the field to the laboratory.Front Immunol. 2023 Jan 12;13:1060985. doi: 10.3389/fimmu.2022.1060985. eCollection 2022. Front Immunol. 2023. PMID: 36713371 Free PMC article. Review.
-
The Hitchhiker Guide to CD4+ T-Cell Depletion in Lentiviral Infection. A Critical Review of the Dynamics of the CD4+ T Cells in SIV and HIV Infection.Front Immunol. 2021 Jul 21;12:695674. doi: 10.3389/fimmu.2021.695674. eCollection 2021. Front Immunol. 2021. PMID: 34367156 Free PMC article.
-
Th17 cells, HIV and the gut mucosal barrier.Curr Opin HIV AIDS. 2010 Mar;5(2):173-8. doi: 10.1097/COH.0b013e328335eda3. Curr Opin HIV AIDS. 2010. PMID: 20543596 Review.
-
African green monkeys avoid SIV disease progression by preventing intestinal dysfunction and maintaining mucosal barrier integrity.PLoS Pathog. 2020 Mar 2;16(3):e1008333. doi: 10.1371/journal.ppat.1008333. eCollection 2020 Mar. PLoS Pathog. 2020. PMID: 32119719 Free PMC article.
-
Longitudinal Examination of the Intestinal Lamina Propria Cellular Compartment of Simian Immunodeficiency Virus-Infected Rhesus Macaques Provides Broader and Deeper Insights into the Link between Aberrant MicroRNA Expression and Persistent Immune Activation.J Virol. 2016 Apr 29;90(10):5003-5019. doi: 10.1128/JVI.00189-16. Print 2016 May 15. J Virol. 2016. PMID: 26937033 Free PMC article.
Cited by
-
Large expansion of plasma commensal viruses is associated with SIV pathogenesis in Macaca leonina.Sci Adv. 2024 Oct 4;10(40):eadq1152. doi: 10.1126/sciadv.adq1152. Epub 2024 Oct 2. Sci Adv. 2024. PMID: 39356751 Free PMC article.
-
Alcohol use predicts elevation in inflammatory marker soluble CD14 in men living with HIV.AIDS Care. 2016 Nov;28(11):1434-40. doi: 10.1080/09540121.2016.1189497. Epub 2016 May 30. AIDS Care. 2016. PMID: 27242060 Free PMC article.
-
Combined effects of HIV and obesity on the gastrointestinal microbiome of young men who have sex with men.HIV Med. 2020 Jul;21(6):365-377. doi: 10.1111/hiv.12838. Epub 2019 Dec 27. HIV Med. 2020. PMID: 31883184 Free PMC article.
-
Walk on the wild side: SIV infection in African non-human primate hosts-from the field to the laboratory.Front Immunol. 2023 Jan 12;13:1060985. doi: 10.3389/fimmu.2022.1060985. eCollection 2022. Front Immunol. 2023. PMID: 36713371 Free PMC article. Review.
-
Microbial Translocation and Immune Activation in HIV-1 Infected Pregnant Women.Curr HIV Res. 2018;16(3):208-215. doi: 10.2174/1570162X16666180731145011. Curr HIV Res. 2018. PMID: 30062968 Free PMC article.
References
-
- Douek DC. 2003. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 5:172–177 - PubMed
-
- Lenardo MJ, Angleman SB, Bounkeua V, Dimas J, Duvall MG, Graubard MB, Hornung F, Selkirk MC, Speirs CK, Trageser C, Orenstein JO, Bolton DL. 2002. Cytopathic killing of peripheral blood CD4(+) T lymphocytes by human immunodeficiency virus type 1 appears necrotic rather than apoptotic and does not require env. J. Virol. 76:5082–5093 - PMC - PubMed
-
- McMichael AJ, Rowland-Jones SL. 2001. Cellular immune responses to HIV. Nature 410:980–987 - PubMed
-
- Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narváez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942–947 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

