Lymphocyte-derived ACh regulates local innate but not adaptive immunity
- PMID: 23297238
- PMCID: PMC3557089
- DOI: 10.1073/pnas.1221655110
Lymphocyte-derived ACh regulates local innate but not adaptive immunity
Erratum in
- Proc Natl Acad Sci U S A.. Olofsson, Peder [corrected to Olofsson, Peder S]
Abstract
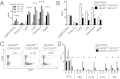
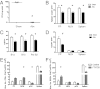
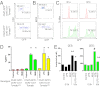
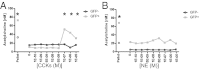
Appropriate control of immune responses is a critical determinant of health. Here, we show that choline acetyltransferase (ChAT) is expressed and ACh is produced by B cells and other immune cells that have an impact on innate immunity. ChAT expression occurs in mucosal-associated lymph tissue, subsequent to microbial colonization, and is reduced by antibiotic treatment. MyD88-dependent Toll-like receptor up-regulates ChAT in a transient manner. Unlike the previously described CD4(+) T-cell population that is stimulated by norepinephrine to release ACh, ChAT(+) B cells release ACh after stimulation with sulfated cholecystokinin but not norepinephrine. ACh-producing B-cells reduce peritoneal neutrophil recruitment during sterile endotoxemia independent of the vagus nerve, without affecting innate immune cell activation. Endothelial cells treated with ACh in vitro reduced endothelial cell adhesion molecule expression in a muscarinic receptor-dependent manner. Despite this ability, ChAT(+) B cells were unable to suppress effector T-cell function in vivo. Therefore, ACh produced by lymphocytes has specific functions, with ChAT(+) B cells controlling the local recruitment of neutrophils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Neuroimmunology: ChATty B cells.Nat Rev Immunol. 2013 Feb;13(2):70. doi: 10.1038/nri3396. Nat Rev Immunol. 2013. PMID: 23348411 No abstract available.
Similar articles
-
Cholinergic macrophages promote the resolution of peritoneal inflammation.Proc Natl Acad Sci U S A. 2024 Jul 2;121(27):e2402143121. doi: 10.1073/pnas.2402143121. Epub 2024 Jun 26. Proc Natl Acad Sci U S A. 2024. PMID: 38923993 Free PMC article.
-
Physiological functions of the cholinergic system in immune cells.J Pharmacol Sci. 2017 May;134(1):1-21. doi: 10.1016/j.jphs.2017.05.002. Epub 2017 May 12. J Pharmacol Sci. 2017. PMID: 28552584 Review.
-
Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit.Science. 2011 Oct 7;334(6052):98-101. doi: 10.1126/science.1209985. Epub 2011 Sep 15. Science. 2011. PMID: 21921156 Free PMC article.
-
Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori.Gastroenterology. 2007 Jul;133(1):150-163.e3. doi: 10.1053/j.gastro.2007.04.071. Epub 2007 May 3. Gastroenterology. 2007. PMID: 17631139
-
Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function.Front Biosci. 2004 Sep 1;9:2063-85. doi: 10.2741/1390. Front Biosci. 2004. PMID: 15353271 Review.
Cited by
-
Modulation of the Immune Response by Nematode Secreted Acetylcholinesterase Revealed by Heterologous Expression in Trypanosoma musculi.PLoS Pathog. 2016 Nov 1;12(11):e1005998. doi: 10.1371/journal.ppat.1005998. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27802350 Free PMC article.
-
B cells modulate lung antiviral inflammatory responses via the neurotransmitter acetylcholine.Res Sq [Preprint]. 2024 Jun 25:rs.3.rs-4421566. doi: 10.21203/rs.3.rs-4421566/v1. Res Sq. 2024. PMID: 38978583 Free PMC article. Preprint.
-
Pathophysiology of Sepsis and Genesis of Septic Shock: The Critical Role of Mesenchymal Stem Cells (MSCs).Int J Mol Sci. 2022 Aug 17;23(16):9274. doi: 10.3390/ijms23169274. Int J Mol Sci. 2022. PMID: 36012544 Free PMC article. Review.
-
Immune status influences fear and anxiety responses in mice after acute stress exposure.Brain Behav Immun. 2014 May;38:192-201. doi: 10.1016/j.bbi.2014.02.001. Epub 2014 Feb 10. Brain Behav Immun. 2014. PMID: 24524915 Free PMC article.
-
A simple method for establishing adherent ex vivo explant cultures from human eye pathologies for use in subsequent calcium imaging and inflammatory studies.J Immunol Res. 2014;2014:232659. doi: 10.1155/2014/232659. Epub 2014 Sep 4. J Immunol Res. 2014. PMID: 25276840 Free PMC article.
References
-
- Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med. 1995;332(1):27–37. - PubMed
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. - PubMed
-
- Ma B, von Wasielewski R, Lindenmaier W, Dittmar KEJ. Immmunohistochemical study of the blood and lymphatic vasculature and the innervation of mouse gut and gut-associated lymphoid tissue. Anat Histol Embryol. 2007;36(1):62–74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

