Extracellular transmission of a DNA mycovirus and its use as a natural fungicide
- PMID: 23297222
- PMCID: PMC3557086
- DOI: 10.1073/pnas.1213755110
Extracellular transmission of a DNA mycovirus and its use as a natural fungicide
Abstract
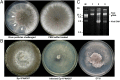
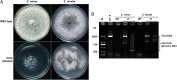
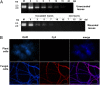
Mycoviruses are thought not to be infectious as free particles and to lack an extracellular phase in their life cycles, limiting the broad use of hypovirulence-associated mycoviruses in controlling fungal disease. Here, we demonstrate that purified particles of a DNA mycovirus, Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1), are infectious when applied extracellularly to its host Sclerotinia sclerotiorum. Virus particles isolated from an infected host can infect the hyphae of virus-free S. sclerotiorum directly when applied to hyphae grown on potato dextrose agar or sprayed on leaves of Arabidopsis thaliana and Brassica napus, regardless of vegetative compatibility affiliation. When applied to leaves, the virus can suppress the development of lesions. SsHADV-1 can also reduce disease severity and enhance rapeseed yield significantly under field conditions. SsHADV-1 has a narrow host range; it can infect Sclerotinia minor and Sclerotinia nivalis, sister species of S. sclerotiorum, and cause debilitation of these two fungi, but cannot infect or transfect other tested fungi, such as Botrytis cinerea, which shares the same family with S. sclerotiorum. Virus particles are likely to be very stable on the leaves of A. thaliana plants because viral DNA could be detected at 15 d postinoculation on unwounded leaves and at 10 d postinoculation on wounded leaves, respectively; however, this virus could not infect and move in plant cells. Our findings may prompt a reconsideration of the generalization that mycoviruses lack an extracellular phase in their life cycles and stimulate the search for other DNA mycoviruses with potential use as natural fungicides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Viruses of the plant pathogenic fungus Sclerotinia sclerotiorum.Adv Virus Res. 2013;86:215-48. doi: 10.1016/B978-0-12-394315-6.00008-8. Adv Virus Res. 2013. PMID: 23498908 Review.
-
Early Transcriptional Response to DNA Virus Infection in Sclerotinia sclerotiorum.Viruses. 2019 Mar 19;11(3):278. doi: 10.3390/v11030278. Viruses. 2019. PMID: 30893849 Free PMC article.
-
A Novel Deltaflexivirus that Infects the Plant Fungal Pathogen, Sclerotinia sclerotiorum, Can Be Transmitted Among Host Vegetative Incompatible Strains.Viruses. 2018 May 31;10(6):295. doi: 10.3390/v10060295. Viruses. 2018. PMID: 29857477 Free PMC article.
-
A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus.Proc Natl Acad Sci U S A. 2010 May 4;107(18):8387-92. doi: 10.1073/pnas.0913535107. Epub 2010 Apr 19. Proc Natl Acad Sci U S A. 2010. PMID: 20404139 Free PMC article.
-
First Report of Mycovirus Infected Sclerotinia sclerotiorum in Cauliflower from Sirmaur District of Himachal Pradesh.Recent Pat Biotechnol. 2020;14(4):283-294. doi: 10.2174/1872208314666200806112116. Recent Pat Biotechnol. 2020. PMID: 32767933 Review.
Cited by
-
Direct Metatranscriptomic Survey of the Sunflower Microbiome and Virome.Viruses. 2021 Sep 18;13(9):1867. doi: 10.3390/v13091867. Viruses. 2021. PMID: 34578448 Free PMC article.
-
Viruses of plant-pathogenic fungi: a promising biocontrol strategy for Sclerotinia sclerotiorum.Arch Microbiol. 2023 Dec 24;206(1):38. doi: 10.1007/s00203-023-03774-8. Arch Microbiol. 2023. PMID: 38142438 Review.
-
Virus Infection of Aspergillus fumigatus Compromises the Fungus in Intermicrobial Competition.Viruses. 2021 Apr 16;13(4):686. doi: 10.3390/v13040686. Viruses. 2021. PMID: 33923408 Free PMC article.
-
Transfection of Sclerotinia sclerotiorum with in vitro transcripts of a naturally occurring interspecific recombinant of Sclerotinia sclerotiorum hypovirus 2 significantly reduces virulence of the fungus.J Virol. 2015 May;89(9):5060-71. doi: 10.1128/JVI.03199-14. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694604 Free PMC article.
-
Human Clinical Isolates of Pathogenic Fungi Are Host to Diverse Mycoviruses.Microbiol Spectr. 2022 Oct 26;10(5):e0161022. doi: 10.1128/spectrum.01610-22. Epub 2022 Aug 22. Microbiol Spectr. 2022. PMID: 35993766 Free PMC article.
References
-
- King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2012.
-
- Nuss DL. Hypovirulence: Mycoviruses at the fungal-plant interface. Nat Rev Microbiol. 2005;3(8):632–642. - PubMed
-
- Ghabrial SA, Suzuki N. Viruses of plant pathogenic fungi. Annu Rev Phytopathol. 2009;47:353–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

