Results of a phase 2 trial of the single-agent histone deacetylase inhibitor panobinostat in patients with relapsed/refractory Waldenström macroglobulinemia
- PMID: 23287861
- PMCID: PMC3578951
- DOI: 10.1182/blood-2012-06-439307
Results of a phase 2 trial of the single-agent histone deacetylase inhibitor panobinostat in patients with relapsed/refractory Waldenström macroglobulinemia
Abstract
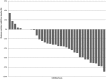
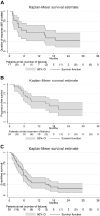
The present study aimed to determine the safety and activity of the histone deacetylase inhibitor panobinostat in patients with relapsed/refractory Waldenström macroglobulinemia (WM). Eligibility criteria included patients with relapsed/refractory WM with any number of prior therapies. Patients received panobinostat at 30 mg 3 times a week; 12 of 36 (33%) patients were enrolled at 25 mg dose. A total of 36 patients received therapy. The median age was 62 years (range, 47-80) and the median number of prior therapies was 3 (range, 1-8). All of the patients had received prior rituximab. Minimal response (MR) or better was achieved in 47% of patients (90% confidence interval [CI], 33-62), with 22% partial remissions and 25% MR. In addition, 18 (50%) patients achieved stable disease and none showed progression while on therapy. The median time to first response was 1.8 months (range, 1.7-3.2). The median progression-free survival was 6.6 months(90% CI, 5.5-14.8). Grade 3 and 4 toxicities included thrombocytopenia (67%), neutropenia (36%), anemia (28%), leukopenia (22%), and fatigue (11%). We conclude that panobinostat is an active therapeutic agent in patients with relapsed/ refractory WM. This study (www.clinicaltrials.gov identifier: NCT00936611) establishes a role for histone deacetylase inhibitors as an active class of therapeutic agents in WM.
Figures
Similar articles
-
Bortezomib, thalidomide, dexamethasone, and panobinostat for patients with relapsed multiple myeloma (MUK-six): a multicentre, open-label, phase 1/2 trial.Lancet Haematol. 2016 Dec;3(12):e572-e580. doi: 10.1016/S2352-3026(16)30165-X. Epub 2016 Nov 12. Lancet Haematol. 2016. PMID: 27843120 Clinical Trial.
-
Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial.Lancet Oncol. 2014 Oct;15(11):1195-206. doi: 10.1016/S1470-2045(14)70440-1. Epub 2014 Sep 18. Lancet Oncol. 2014. PMID: 25242045 Clinical Trial.
-
A phase I trial of panobinostat and epirubicin in solid tumors with a dose expansion in patients with sarcoma.Ann Oncol. 2016 May;27(5):947-52. doi: 10.1093/annonc/mdw044. Epub 2016 Feb 21. Ann Oncol. 2016. PMID: 26903311 Free PMC article. Clinical Trial.
-
Panobinostat: a novel pan-deacetylase inhibitor for the treatment of relapsed or relapsed and refractory multiple myeloma.Expert Rev Anticancer Ther. 2015;15(7):737-48. doi: 10.1586/14737140.2015.1047770. Epub 2015 Jun 7. Expert Rev Anticancer Ther. 2015. PMID: 26051506 Review.
-
Panobinostat: A histone deacetylase inhibitor for the treatment of relapsed or refractory multiple myeloma.Am J Health Syst Pharm. 2016 Apr 1;73(7):441-50. doi: 10.2146/ajhp150487. Am J Health Syst Pharm. 2016. PMID: 27001985 Review.
Cited by
-
Molecular Mechanism of the Cell Death Induced by the Histone Deacetylase Pan Inhibitor LBH589 (Panobinostat) in Wilms Tumor Cells.PLoS One. 2015 Jul 15;10(7):e0126566. doi: 10.1371/journal.pone.0126566. eCollection 2015. PLoS One. 2015. PMID: 26176219 Free PMC article.
-
Nucleic Acid Biomarkers in Waldenström Macroglobulinemia and IgM-MGUS: Current Insights and Clinical Relevance.Diagnostics (Basel). 2022 Apr 12;12(4):969. doi: 10.3390/diagnostics12040969. Diagnostics (Basel). 2022. PMID: 35454017 Free PMC article. Review.
-
Histone deacetylase 1 plays a predominant pro-oncogenic role in Eμ-myc driven B cell lymphoma.Sci Rep. 2016 Nov 25;6:37772. doi: 10.1038/srep37772. Sci Rep. 2016. PMID: 27886239 Free PMC article.
-
How to manage Waldenstrom's macroglobulinemia.Leukemia. 2013 Apr;27(4):762-72. doi: 10.1038/leu.2013.36. Epub 2013 Feb 6. Leukemia. 2013. PMID: 23385376 Free PMC article. Review.
-
Marine-derived angiogenesis inhibitors for cancer therapy.Mar Drugs. 2013 Mar 15;11(3):903-33. doi: 10.3390/md11030903. Mar Drugs. 2013. PMID: 23502698 Free PMC article. Review.
References
-
- Dimopoulos MA, Panayiotidis P, Moulopoulos LA, Sfikakis P, Dalakas M. Waldenstrom's macroglobulinemia: clinical features, complications, and management. J Clin Oncol. 2000;18(1):214–226. - PubMed
-
- Dimopoulos MA, Kyle RA, Anagnostopoulos A, Treon SP. Diagnosis and management of Waldenstrom's macroglobulinemia. J Clin Oncol. 2005;23(7):1564–1577. - PubMed
-
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30(2):110–115. - PubMed
-
- Ghobrial IM, Gertz MA, Fonseca R. Waldenstrom macroglobulinaemia. Lancet Oncol. 2003;4(11):679–685. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

