TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment
- PMID: 23277487
- PMCID: PMC3552130
- DOI: 10.4049/jimmunol.1202659
TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment
Abstract
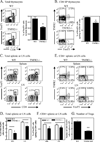
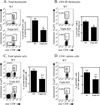
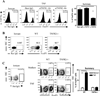
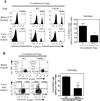
Several lines of evidence indicate the instability of CD4(+)Foxp3(+) regulatory T cells (Tregs). We have therefore investigated means of promoting the stability of Tregs. In this study, we found that the proportion of Tregs in mouse strains deficient in TNFR2 or its ligands was reduced in the thymus and peripheral lymphoid tissues, suggesting a potential role of TNFR2 in promoting the sustained expression of Foxp3. We observed that upon in vitro activation with plate-bound anti-CD3 Ab and soluble anti-CD28 Ab, Foxp3 expression by highly purified mouse Tregs was markedly downregulated. Importantly, TNF partially abrogated this effect of TCR stimulation and stabilized Foxp3 expression. This effect of TNF was blocked by anti-TNFR2 Ab, but not by anti-TNFR1 Ab. Furthermore, TNF was not able to maintain Foxp3 expression by TNFR2-deficient Tregs. In a mouse colitis model induced by transfer of naive CD4 cells into Rag1(-/-) mice, the disease could be inhibited by cotransfer of wild-type Tregs, but not by cotransfer of TNFR2-deficient Tregs. Furthermore, in the lamina propria of the colitis model, most wild-type Tregs maintained Foxp3 expression. In contrast, an increased number of TNFR2-deficient Tregs lost Foxp3 expression. Thus, our data clearly show that TNFR2 is critical for the phenotypic and functional stability of Tregs in the inflammatory environment. This effect of TNF should be taken into account when designing future therapy of autoimmunity and graft-versus-host disease by using TNF inhibitors.
Figures



Similar articles
-
In vitro generated Th17 cells support the expansion and phenotypic stability of CD4(+)Foxp3(+) regulatory T cells in vivo.Cytokine. 2014 Jan;65(1):56-64. doi: 10.1016/j.cyto.2013.09.008. Epub 2013 Sep 27. Cytokine. 2014. PMID: 24080164 Free PMC article.
-
Transient local depletion of Foxp3+ regulatory T cells during recovery from colitis via Fas/Fas ligand-induced death.J Immunol. 2008 Jun 15;180(12):8316-26. doi: 10.4049/jimmunol.180.12.8316. J Immunol. 2008. PMID: 18523298
-
TNFR2 expression by CD4 effector T cells is required to induce full-fledged experimental colitis.Sci Rep. 2016 Sep 7;6:32834. doi: 10.1038/srep32834. Sci Rep. 2016. PMID: 27601345 Free PMC article.
-
TNF-alpha: an activator of CD4+FoxP3+TNFR2+ regulatory T cells.Curr Dir Autoimmun. 2010;11:119-34. doi: 10.1159/000289201. Epub 2010 Feb 18. Curr Dir Autoimmun. 2010. PMID: 20173391 Free PMC article. Review.
-
The Significance of Tumor Necrosis Factor Receptor Type II in CD8+ Regulatory T Cells and CD8+ Effector T Cells.Front Immunol. 2018 Mar 22;9:583. doi: 10.3389/fimmu.2018.00583. eCollection 2018. Front Immunol. 2018. PMID: 29623079 Free PMC article. Review.
Cited by
-
Infliximab therapy balances regulatory T cells, tumour necrosis factor receptor 2 (TNFR2) expression and soluble TNFR2 in sarcoidosis.Clin Exp Immunol. 2016 Aug;185(2):263-70. doi: 10.1111/cei.12808. Epub 2016 Jul 12. Clin Exp Immunol. 2016. PMID: 27158798 Free PMC article.
-
TNFR2 expression predicts the responses to immune checkpoint inhibitor treatments.Front Immunol. 2023 Feb 14;14:1097090. doi: 10.3389/fimmu.2023.1097090. eCollection 2023. Front Immunol. 2023. PMID: 36865537 Free PMC article.
-
Innate and Adaptive Immunity in Noninfectious Granulomatous Lung Disease.J Immunol. 2022 Apr 15;208(8):1835-1843. doi: 10.4049/jimmunol.2101159. J Immunol. 2022. PMID: 35418504 Free PMC article. Review.
-
Role of Cytokines in Thymic Regulatory T Cell Generation: Overview and Updates.Front Immunol. 2022 Mar 31;13:883560. doi: 10.3389/fimmu.2022.883560. eCollection 2022. Front Immunol. 2022. PMID: 35432378 Free PMC article. Review.
-
The Role of TNFR2 and DR3 in the In Vivo Expansion of Tregs in T Cell Depleting Transplantation Regimens.Int J Mol Sci. 2020 May 9;21(9):3347. doi: 10.3390/ijms21093347. Int J Mol Sci. 2020. PMID: 32397343 Free PMC article. Review.
References
-
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. - PubMed
-
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. - PubMed
-
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. - PubMed
-
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

