GSK3β phosphorylates newly identified site in the proline-alanine-rich region of cardiac myosin-binding protein C and alters cross-bridge cycling kinetics in human: short communication
- PMID: 23277198
- PMCID: PMC3595322
- DOI: 10.1161/CIRCRESAHA.112.275602
GSK3β phosphorylates newly identified site in the proline-alanine-rich region of cardiac myosin-binding protein C and alters cross-bridge cycling kinetics in human: short communication
Abstract
Rationale: Cardiac myosin-binding protein C (cMyBP-C) regulates cross-bridge cycling kinetics and, thereby, fine-tunes the rate of cardiac muscle contraction and relaxation. Its effects on cardiac kinetics are modified by phosphorylation. Three phosphorylation sites (Ser275, Ser284, and Ser304) have been identified in vivo, all located in the cardiac-specific M-domain of cMyBP-C. However, recent work has shown that up to 4 phosphate groups are present in human cMyBP-C.
Objective: To identify and characterize additional phosphorylation sites in human cMyBP-C.
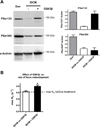
Methods and results: Cardiac MyBP-C was semipurified from human heart tissue. Tandem mass spectrometry analysis identified a novel phosphorylation site on serine 133 in the proline-alanine-rich linker sequence between the C0 and C1 domains of cMyBP-C. Unlike the known sites, Ser133 was not a target of protein kinase A. In silico kinase prediction revealed glycogen synthase kinase 3β (GSK3β) as the most likely kinase to phosphorylate Ser133. In vitro incubation of the C0C2 fragment of cMyBP-C with GSK3β showed phosphorylation on Ser133. In addition, GSK3β phosphorylated Ser304, although the degree of phosphorylation was less compared with protein kinase A-induced phosphorylation at Ser304. GSK3β treatment of single membrane-permeabilized human cardiomyocytes significantly enhanced the maximal rate of tension redevelopment.
Conclusions: GSK3β phosphorylates cMyBP-C on a novel site, which is positioned in the proline-alanine-rich region and increases kinetics of force development, suggesting a noncanonical role for GSK3β at the sarcomere level. Phosphorylation of Ser133 in the linker domain of cMyBP-C may be a novel mechanism to regulate sarcomere kinetics.
Figures



Similar articles
-
Normal cardiac contraction in mice lacking the proline-alanine rich region and C1 domain of cardiac myosin binding protein C.J Mol Cell Cardiol. 2015 Nov;88:124-32. doi: 10.1016/j.yjmcc.2015.09.006. Epub 2015 Oct 8. J Mol Cell Cardiol. 2015. PMID: 26455481 Free PMC article.
-
Identification of novel protein kinase A phosphorylation sites in the M-domain of human and murine cardiac myosin binding protein-C using mass spectrometry analysis.J Proteome Res. 2010 Apr 5;9(4):1843-53. doi: 10.1021/pr901006h. J Proteome Res. 2010. PMID: 20151718 Free PMC article.
-
Activation of myocardial contraction by the N-terminal domains of myosin binding protein-C.Circ Res. 2006 May 26;98(10):1290-8. doi: 10.1161/01.RES.0000222059.54917.ef. Epub 2006 Apr 13. Circ Res. 2006. PMID: 16614305
-
Species-specific differences in the Pro-Ala rich region of cardiac myosin binding protein-C.J Muscle Res Cell Motil. 2009 Dec;30(7-8):303-6. doi: 10.1007/s10974-010-9207-8. Epub 2010 Mar 9. J Muscle Res Cell Motil. 2009. PMID: 20217194 Free PMC article. Review.
-
Cardiac myosin binding protein-C as a central target of cardiac sarcomere signaling: a special mini review series.Pflugers Arch. 2014 Feb;466(2):195-200. doi: 10.1007/s00424-013-1396-8. Epub 2013 Nov 7. Pflugers Arch. 2014. PMID: 24196566 Free PMC article. Review.
Cited by
-
Cardiac myosin-binding protein C: hypertrophic cardiomyopathy mutations and structure-function relationships.Pflugers Arch. 2014 Feb;466(2):201-6. doi: 10.1007/s00424-013-1400-3. Epub 2013 Nov 17. Pflugers Arch. 2014. PMID: 24240729 Review.
-
Cardiac myosin binding protein C phosphorylation affects cross-bridge cycle's elementary steps in a site-specific manner.PLoS One. 2014 Nov 24;9(11):e113417. doi: 10.1371/journal.pone.0113417. eCollection 2014. PLoS One. 2014. PMID: 25420047 Free PMC article.
-
Cardiac myosin binding protein-C Ser302 phosphorylation regulates cardiac β-adrenergic reserve.Sci Adv. 2017 Mar 10;3(3):e1602445. doi: 10.1126/sciadv.1602445. eCollection 2017 Mar. Sci Adv. 2017. PMID: 28345052 Free PMC article.
-
Heat shock proteins and small nucleolar RNAs are dysregulated in a Drosophila model for feline hypertrophic cardiomyopathy.G3 (Bethesda). 2021 Jan 18;11(1):jkaa014. doi: 10.1093/g3journal/jkaa014. G3 (Bethesda). 2021. PMID: 33561224 Free PMC article.
-
Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations.Circ Res. 2013 May 24;112(11):1491-505. doi: 10.1161/CIRCRESAHA.111.300436. Epub 2013 Mar 18. Circ Res. 2013. PMID: 23508784 Free PMC article.
References
-
- Stelzer JE, Dunning SB, Moss RL. Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium. Circ Res. 2006;98:1212–1218. - PubMed
-
- Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res. 2006;99:884–890. - PubMed
-
- Copeland O, Sadayappan S, Messer AE, Steinen GJ, van der Velden J, Marston SB. Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J Mol Cell Cardiol. 2010;49:1003–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

