Energetic coupling between an oxidizable cysteine and the phosphorylatable N-terminus of human liver pyruvate kinase
- PMID: 23270483
- PMCID: PMC3572911
- DOI: 10.1021/bi301341r
Energetic coupling between an oxidizable cysteine and the phosphorylatable N-terminus of human liver pyruvate kinase
Abstract
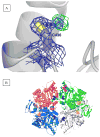
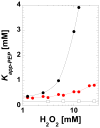
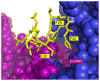
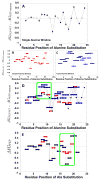
During our efforts to characterize the regulatory properties of human liver pyruvate kinase (L-PYK), we have noted that the affinity of the protein for phosphoenolpyruvate (PEP) becomes reduced several days after cell lysis. A 1.8 Å crystallographic structure of L-PYK with the S12D mimic of phosphorylation indicates that Cys436 is oxidized, the first potential insight into explaining the effect of "aging". Interestingly, the oxidation is only to sulfenic acid despite the crystal growth time period of 2 weeks. Mutagenesis confirms that the side chain of residue 436 is energetically coupled to PEP binding. Mass spectrometry confirms that the oxidation is present in solution and is not an artifact caused by X-ray exposure. Exposure of the L-PYK mutations to H₂O₂ also confirms that PEP affinity is sensitive to the nature of the side chain at position 436. A 1.95 Å structure of the C436M mutant of L-PYK, the only mutation at position 436 that has been shown to strengthen PEP affinity, revealed that the methionine substitution results in the ordering of several N-terminal residues that have not been ordered in previous structures. This result allowed speculation that oxidation of Cys436 and phosphorylation of the N-terminus at Ser12 may function through a similar mechanism, namely the interruption of an activating interaction between the nonphosphorylated N-terminus with the nonoxidized main body of the protein. Mutant cycles were used to provide evidence that mutations of Cys436 are energetically synergistic with N-terminal modifications, a result that is consistent with phosphorylation of the N-terminus and oxidation of Cys436 functioning through mechanisms with common features. Alanine-scanning mutagenesis was used to confirm that the newly ordered N-terminal residues were important to the regulation of enzyme function by the N-terminus of the enzyme (i.e., not an artifact caused by the introduced methionine substitution) and to further define which residues in the N-terminus are energetically coupled to PEP affinity. Collectively, these studies indicate energetic coupling (and potentially mechanistic similarities) between the oxidation of Cys436 and phosphorylation of Ser12 in the N-terminus of L-PYK.
Figures

Similar articles
-
An activating interaction between the unphosphorylated n-terminus of human liver pyruvate kinase and the main body of the protein is interrupted by phosphorylation.Biochemistry. 2009 May 12;48(18):3816-8. doi: 10.1021/bi900421f. Biochemistry. 2009. PMID: 19320443 Free PMC article.
-
Allosteric regulation of human liver pyruvate kinase by peptides that mimic the phosphorylated/dephosphorylated N-terminus.Methods Mol Biol. 2012;796:335-49. doi: 10.1007/978-1-61779-334-9_18. Methods Mol Biol. 2012. PMID: 22052499 Free PMC article.
-
Probing L-pyruvate kinase regulatory phosphorylation site by mutagenesis.Protein J. 2012 Oct;31(7):592-7. doi: 10.1007/s10930-012-9438-1. Protein J. 2012. PMID: 22878931
-
Rheostatic contributions to protein stability can obscure a position's functional role.Protein Sci. 2024 Jul;33(7):e5075. doi: 10.1002/pro.5075. Protein Sci. 2024. PMID: 38895978
-
Effector analogues detect varied allosteric roles for conserved protein-effector interactions in pyruvate kinase isozymes.Biochemistry. 2011 Mar 22;50(11):1934-9. doi: 10.1021/bi200052e. Epub 2011 Feb 14. Biochemistry. 2011. PMID: 21261284 Free PMC article.
Cited by
-
The redox biochemistry of protein sulfenylation and sulfinylation.J Biol Chem. 2013 Sep 13;288(37):26480-8. doi: 10.1074/jbc.R113.467738. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861405 Free PMC article. Review.
-
Rheostats, toggles, and neutrals, Oh my! A new framework for understanding how amino acid changes modulate protein function.J Biol Chem. 2024 Mar;300(3):105736. doi: 10.1016/j.jbc.2024.105736. Epub 2024 Feb 8. J Biol Chem. 2024. PMID: 38336297 Free PMC article. Review.
-
Functional tunability from a distance: Rheostat positions influence allosteric coupling between two distant binding sites.Sci Rep. 2019 Nov 18;9(1):16957. doi: 10.1038/s41598-019-53464-z. Sci Rep. 2019. PMID: 31740686 Free PMC article.
-
Distinct Hepatic PKA and CDK Signaling Pathways Control Activity-Independent Pyruvate Kinase Phosphorylation and Hepatic Glucose Production.Cell Rep. 2019 Dec 10;29(11):3394-3404.e9. doi: 10.1016/j.celrep.2019.11.009. Cell Rep. 2019. PMID: 31825824 Free PMC article.
-
PYK-SubstitutionOME: an integrated database containing allosteric coupling, ligand affinity and mutational, structural, pathological, bioinformatic and computational information about pyruvate kinase isozymes.Database (Oxford). 2023 May 3;2023:baad030. doi: 10.1093/database/baad030. Database (Oxford). 2023. PMID: 37171062 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

