Cab45 is required for Ca(2+)-dependent secretory cargo sorting at the trans-Golgi network
- PMID: 23266954
- PMCID: PMC3529532
- DOI: 10.1083/jcb.201207180
Cab45 is required for Ca(2+)-dependent secretory cargo sorting at the trans-Golgi network
Abstract
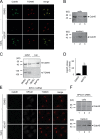
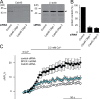
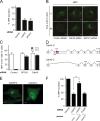
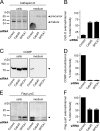
Ca(2+) import into the lumen of the trans-Golgi network (TGN) by the secretory pathway calcium ATPase1 (SPCA1) is required for the sorting of secretory cargo. How is Ca(2+) retained in the lumen of the Golgi, and what is its role in cargo sorting? We show here that a soluble, lumenal Golgi resident protein, Cab45, is required for SPCA1-dependent Ca(2+) import into the TGN; it binds secretory cargo in a Ca(2+)-dependent reaction and is required for its sorting at the TGN.
Figures
Similar articles
-
Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network.J Cell Biol. 2016 May 9;213(3):305-14. doi: 10.1083/jcb.201601089. Epub 2016 May 2. J Cell Biol. 2016. PMID: 27138253 Free PMC article.
-
Cab45-Unraveling key features of a novel secretory cargo sorter at the trans-Golgi network.Eur J Cell Biol. 2017 Aug;96(5):383-390. doi: 10.1016/j.ejcb.2017.03.001. Epub 2017 Mar 18. Eur J Cell Biol. 2017. PMID: 28372832 Review.
-
Exploring new routes for secretory protein export from the trans-Golgi network.Mol Biol Cell. 2018 Feb 1;29(3):235-240. doi: 10.1091/mbc.E17-02-0117. Mol Biol Cell. 2018. PMID: 29382805 Free PMC article.
-
Activity of the SPCA1 Calcium Pump Couples Sphingomyelin Synthesis to Sorting of Secretory Proteins in the Trans-Golgi Network.Dev Cell. 2018 Nov 19;47(4):464-478.e8. doi: 10.1016/j.devcel.2018.10.012. Epub 2018 Nov 1. Dev Cell. 2018. PMID: 30393074 Free PMC article.
-
Secretory cargo sorting at the trans-Golgi network.Trends Cell Biol. 2014 Oct;24(10):584-93. doi: 10.1016/j.tcb.2014.04.007. Epub 2014 May 16. Trends Cell Biol. 2014. PMID: 24841758 Review.
Cited by
-
Vti1a/b regulate synaptic vesicle and dense core vesicle secretion via protein sorting at the Golgi.Nat Commun. 2018 Aug 24;9(1):3421. doi: 10.1038/s41467-018-05699-z. Nat Commun. 2018. PMID: 30143604 Free PMC article.
-
Receptor-mediated transport of vacuolar proteins: a critical analysis and a new model.Protoplasma. 2014 Jan;251(1):247-64. doi: 10.1007/s00709-013-0542-7. Epub 2013 Sep 10. Protoplasma. 2014. PMID: 24019013
-
Molecular mechanisms of polarized transport to the apical plasma membrane.Front Cell Dev Biol. 2024 Sep 26;12:1477173. doi: 10.3389/fcell.2024.1477173. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39445332 Free PMC article. Review.
-
Lipid-dependent coupling of secretory cargo sorting and trafficking at the trans-Golgi network.FEBS Lett. 2019 Sep;593(17):2412-2427. doi: 10.1002/1873-3468.13552. Epub 2019 Jul 30. FEBS Lett. 2019. PMID: 31344259 Free PMC article. Review.
-
The Trans Golgi Region is a Labile Intracellular Ca2+ Store Sensitive to Emetine.Sci Rep. 2018 Nov 21;8(1):17143. doi: 10.1038/s41598-018-35280-z. Sci Rep. 2018. PMID: 30464185 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

