Novel 3D analysis of Claudin-5 reveals significant endothelial heterogeneity among CNS microvessels
- PMID: 23261753
- PMCID: PMC3570614
- DOI: 10.1016/j.mvr.2012.12.001
Novel 3D analysis of Claudin-5 reveals significant endothelial heterogeneity among CNS microvessels
Abstract
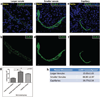
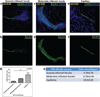
Tight junctions (TJs) feature critically in maintaining the integrity of the blood-brain barrier (BBB), and undergo significant disruption during neuroinflammatory diseases. Accordingly, the expression and distribution of CLN-5, a prominent TJ protein in central nervous system (CNS) microvessels and BBB determinant, has been shown to parallel physiological and pathophysiological changes in microvascular function. However, efforts to quantify CLN-5 within the CNS microvasculature in situ, by using conventional two-dimensional immunohistochemical analysis of thin sections, are encumbered by the tortuosity of capillaries and distorted diameters of inflamed venules. Herein, we describe a novel contour-based 3D image visualization and quantification method, employing high-resolution confocal z-stacks from thick immunofluorescently-stained thoraco-lumbar spinal cord cryosections, to analyze CLN-5 along the junctional regions of different-sized CNS microvascular segments. Analysis was performed on spinal cords of both healthy mice, and mice experiencing experimental autoimmune encephalomyelitis (EAE), an animal model of the neuroinflammatory disease multiple sclerosis. Results indicated that, under normal conditions, the density of CLN-5 staining (CLN-5 intensity/ endothelial surface area) was greatest in the capillaries and smaller venules, and least in the larger venules. This heterogeneity in junctional CLN-5 staining was exacerbated during EAE, as spinal venules revealed a significant loss of junctional CLN-5 staining that was associated with focal leukocyte extravasation, while adjacent capillaries exhibited neither CLN-5 loss nor infiltrating leukocytes. However, despite only venules displaying these behaviors, both capillaries and venules evidenced leakage of IgG during disease, further underscoring the heterogeneity of the inflammatory response in CNS microvessels. This method should be readily adaptable to analyzing other junctional proteins of the CNS and peripheral microvasculature, and serve to highlight their role(s) in health and disease.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Appearance of claudin-5+ leukocytes in the central nervous system during neuroinflammation: a novel role for endothelial-derived extracellular vesicles.J Neuroinflammation. 2016 Nov 16;13(1):292. doi: 10.1186/s12974-016-0755-8. J Neuroinflammation. 2016. PMID: 27852330 Free PMC article.
-
Appearance of claudin-5+ leukocyte subtypes in the blood and CNS during progression of EAE.J Neuroinflammation. 2021 Dec 21;18(1):296. doi: 10.1186/s12974-021-02328-3. J Neuroinflammation. 2021. PMID: 34933669 Free PMC article.
-
Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation.J Neuroinflammation. 2014 Jan 21;11:10. doi: 10.1186/1742-2094-11-10. J Neuroinflammation. 2014. PMID: 24444311 Free PMC article.
-
Molecular mechanisms involved in T cell migration across the blood-brain barrier.J Neural Transm (Vienna). 2006 Apr;113(4):477-85. doi: 10.1007/s00702-005-0409-y. J Neural Transm (Vienna). 2006. PMID: 16550326 Review.
-
Heterogeneity of the blood-brain barrier.Tissue Barriers. 2016 Jan 28;4(1):e1143544. doi: 10.1080/21688370.2016.1143544. eCollection 2016 Jan-Mar. Tissue Barriers. 2016. PMID: 27141424 Free PMC article. Review.
Cited by
-
Preservation of spatial memory and neuroprotection by the fatty acid amide hydrolase inhibitor URB597 in a rat model of vascular dementia.Ann Transl Med. 2021 Feb;9(3):228. doi: 10.21037/atm-20-4431. Ann Transl Med. 2021. PMID: 33708855 Free PMC article.
-
Changes in blood-spinal cord barrier permeability and neuroimmune interactions in the underlying mechanisms of chronic pain.Pain Rep. 2021 Mar 9;6(1):e879. doi: 10.1097/PR9.0000000000000879. eCollection 2021. Pain Rep. 2021. PMID: 33981925 Free PMC article. Review.
-
How air pollution alters brain development: the role of neuroinflammation.Transl Neurosci. 2016 Mar 21;7(1):24-30. doi: 10.1515/tnsci-2016-0005. eCollection 2016. Transl Neurosci. 2016. PMID: 28123818 Free PMC article. Review.
-
CD8+ T Cells Induce Fatal Brainstem Pathology during Cerebral Malaria via Luminal Antigen-Specific Engagement of Brain Vasculature.PLoS Pathog. 2016 Dec 1;12(12):e1006022. doi: 10.1371/journal.ppat.1006022. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27907215 Free PMC article.
-
Loss of IGF1R in Human Astrocytes Alters Complex I Activity and Support for Neurons.Neuroscience. 2018 Oct 15;390:46-59. doi: 10.1016/j.neuroscience.2018.07.029. Epub 2018 Jul 27. Neuroscience. 2018. PMID: 30056117 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

