Metabolism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by mitochondrion-targeted cytochrome P450 2D6: implications in Parkinson disease
- PMID: 23258538
- PMCID: PMC3567693
- DOI: 10.1074/jbc.M112.402123
Metabolism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by mitochondrion-targeted cytochrome P450 2D6: implications in Parkinson disease
Abstract
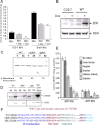
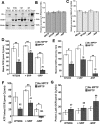
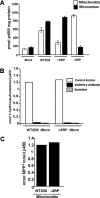
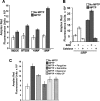
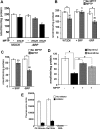
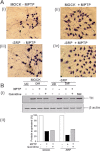
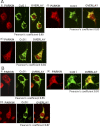
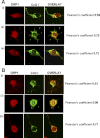
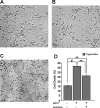
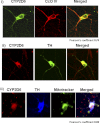
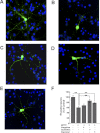
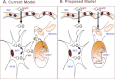
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a neurotoxic side product formed in the chemical synthesis of desmethylprodine opioid analgesic, which induces Parkinson disease. Monoamine oxidase B, present in the mitochondrial outer membrane of glial cells, catalyzes the oxidation of MPTP to the toxic 1-methyl-4-phenylpyridinium ion (MPP(+)), which then targets the dopaminergic neurons causing neuronal death. Here, we demonstrate that mitochondrion-targeted human cytochrome P450 2D6 (CYP2D6), supported by mitochondrial adrenodoxin and adrenodoxin reductase, can efficiently catalyze the metabolism of MPTP to MPP(+), as shown with purified enzymes and also in cells expressing mitochondrial CYP2D6. Neuro-2A cells stably expressing predominantly mitochondrion-targeted CYP2D6 were more sensitive to MPTP-mediated mitochondrial respiratory dysfunction and complex I inhibition than cells expressing predominantly endoplasmic reticulum-targeted CYP2D6. Mitochondrial CYP2D6 expressing Neuro-2A cells produced higher levels of reactive oxygen species and showed abnormal mitochondrial structures. MPTP treatment also induced mitochondrial translocation of an autophagic marker, Parkin, and a mitochondrial fission marker, Drp1, in differentiated neurons expressing mitochondrial CYP2D6. MPTP-mediated toxicity in primary dopaminergic neurons was attenuated by CYP2D6 inhibitor, quinidine, and also partly by monoamine oxidase B inhibitors deprenyl and pargyline. These studies show for the first time that dopaminergic neurons expressing mitochondrial CYP2D6 are fully capable of activating the pro-neurotoxin MPTP and inducing neuronal damage, which is effectively prevented by the CYP2D6 inhibitor quinidine.
Figures

Similar articles
-
Mitochondrially targeted cytochrome P450 2D6 is involved in monomethylamine-induced neuronal damage in mouse models.J Biol Chem. 2019 Jun 28;294(26):10336-10348. doi: 10.1074/jbc.RA119.008848. Epub 2019 May 20. J Biol Chem. 2019. PMID: 31113867 Free PMC article.
-
Comparative aromatic hydroxylation and N-demethylation of MPTP neurotoxin and its analogs, N-methylated beta-carboline and isoquinoline alkaloids, by human cytochrome P450 2D6.Toxicol Appl Pharmacol. 2006 Nov 1;216(3):387-98. doi: 10.1016/j.taap.2006.06.003. Epub 2006 Jun 15. Toxicol Appl Pharmacol. 2006. PMID: 16870220
-
The Essential Role of Drp1 and Its Regulation by S-Nitrosylation of Parkin in Dopaminergic Neurodegeneration: Implications for Parkinson's Disease.Antioxid Redox Signal. 2016 Oct 10;25(11):609-622. doi: 10.1089/ars.2016.6634. Epub 2016 Aug 8. Antioxid Redox Signal. 2016. PMID: 27267045
-
Amphetamine-metabolites of deprenyl involved in protection against neurotoxicity induced by MPTP and 2'-methyl-MPTP.J Neural Transm Suppl. 1994;41:207-19. doi: 10.1007/978-3-7091-9324-2_27. J Neural Transm Suppl. 1994. PMID: 7931228 Review.
-
MPTP as a mitochondrial neurotoxic model of Parkinson's disease.J Bioenerg Biomembr. 2004 Aug;36(4):375-9. doi: 10.1023/B:JOBB.0000041771.66775.d5. J Bioenerg Biomembr. 2004. PMID: 15377875 Review.
Cited by
-
Roles of selected non-P450 human oxidoreductase enzymes in protective and toxic effects of chemicals: review and compilation of reactions.Arch Toxicol. 2022 Aug;96(8):2145-2246. doi: 10.1007/s00204-022-03304-3. Epub 2022 Jun 1. Arch Toxicol. 2022. PMID: 35648190 Free PMC article. Review.
-
Elucidating the Neuroprotective Role of PPARs in Parkinson's Disease: A Neoteric and Prospective Target.Int J Mol Sci. 2021 Sep 21;22(18):10161. doi: 10.3390/ijms221810161. Int J Mol Sci. 2021. PMID: 34576325 Free PMC article. Review.
-
MPTP neurotoxicity is highly concordant between the sexes among BXD recombinant inbred mouse strains.Neurotoxicology. 2016 Jul;55:40-47. doi: 10.1016/j.neuro.2016.04.008. Epub 2016 May 12. Neurotoxicology. 2016. PMID: 27182044 Free PMC article.
-
Curcumin-Activated Mesenchymal Stem Cells Derived from Human Umbilical Cord and Their Effects on MPTP-Mouse Model of Parkinson's Disease: A New Biological Therapy for Parkinson's Disease.Stem Cells Int. 2020 Feb 17;2020:4636397. doi: 10.1155/2020/4636397. eCollection 2020. Stem Cells Int. 2020. PMID: 32148518 Free PMC article.
-
Unusual cytochrome p450 enzymes and reactions.J Biol Chem. 2013 Jun 14;288(24):17065-73. doi: 10.1074/jbc.R113.462275. Epub 2013 Apr 30. J Biol Chem. 2013. PMID: 23632016 Free PMC article. Review.
References
-
- Kopin I. J., Markey S. P. (1988) MPTP toxicity. Implications for research in Parkinson disease. Annu. Rev. Neurosci. 11, 81–96 - PubMed
-
- Sonsalla P. K., Heikkila R. E. (1986) The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur. J. Pharmacol. 129, 339–345 - PubMed
-
- Chiba K., Trevor A., Castagnoli N., Jr. (1984) Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem. Biophys. Res. Commun. 120, 574–578 - PubMed
-
- Chiba K., Peterson L. A., Castagnoli K. P., Trevor A. J., Castagnoli N., Jr. (1985) Studies on the molecular mechanism of bioactivation of the selective nigrostriatal toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Drug Metab. Dispos. 13, 342–347 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

