A novel function of MUC18: amplification of lung inflammation during bacterial infection
- PMID: 23256918
- PMCID: PMC3586690
- DOI: 10.1016/j.ajpath.2012.11.005
A novel function of MUC18: amplification of lung inflammation during bacterial infection
Abstract
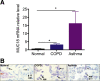
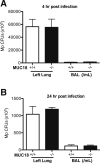
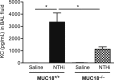
Bacterial infection plays a critical role in exacerbations of various lung diseases, including chronic pulmonary obstructive disease (COPD) and asthma. Excessive lung inflammation is a prominent feature in disease exacerbations, but the underlying mechanisms remain poorly understood. Cell surface glycoprotein MUC18 (alias CD146 or melanoma cell adhesion molecule) has been shown to promote metastasis in several tumors, including melanoma. We explored the function of MUC18 in lung inflammatory responses to bacteria (eg, Mycoplasma pneumoniae) involved in lung disease exacerbations. MUC18 expression was increased in alveolar macrophages from lungs of COPD and asthma patients, compared with normal healthy human subjects. Mouse alveolar macrophages also express MUC18. After M. pneumoniae lung infection, Muc18(-/-) mice exhibited lower levels of the lung proinflammatory cytokines KC and TNF-α and less neutrophil recruitment than Muc18(+/+) mice. Alveolar macrophages from Muc18(-/-) mice produced less KC than those from Muc18(+/+) mice. In Muc18(-/-) mouse alveolar macrophages, adenovirus-mediated MUC18 gene transfer increased KC production. MUC18 amplified proinflammatory responses in alveolar macrophages, in part through enhancing the activation of nuclear factor-κB (NF-κB). Our results demonstrate, for the first time, that MUC18 exerts a proinflammatory function during lung bacterial infection. Up-regulated MUC18 expression in lungs (eg, in alveolar macrophages) of COPD and asthma patients may contribute to excessive inflammation during disease exacerbations.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

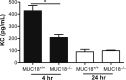

Similar articles
-
Heat shock factor 1 protects against lung mycoplasma pneumoniae infection in mice.J Innate Immun. 2012;4(1):59-68. doi: 10.1159/000333089. Epub 2011 Oct 26. J Innate Immun. 2012. PMID: 22042134 Free PMC article.
-
Up-regulation of MUC18 in airway epithelial cells by IL-13: implications in bacterial adherence.Am J Respir Cell Mol Biol. 2011 May;44(5):606-13. doi: 10.1165/rcmb.2010-0384OC. Epub 2011 Jan 14. Am J Respir Cell Mol Biol. 2011. PMID: 21239604 Free PMC article.
-
Neutrophil-Mediated Lung Injury Both via TLR2-Dependent Production of IL-1α and IL-12 p40, and TLR2-Independent CARDS Toxin after Mycoplasma pneumoniae Infection in Mice.Microbiol Spectr. 2021 Dec 22;9(3):e0158821. doi: 10.1128/spectrum.01588-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34937175 Free PMC article.
-
Inflammatory cells and chronic obstructive pulmonary disease.Curr Drug Targets Inflamm Allergy. 2005 Dec;4(6):607-18. doi: 10.2174/156801005774912824. Curr Drug Targets Inflamm Allergy. 2005. PMID: 17305517 Review.
-
Signal transduction pathways linking the activation of alveolar macrophages with the recruitment of neutrophils to lungs in chronic obstructive pulmonary disease.Exp Lung Res. 2009 Aug;35(6):439-85. doi: 10.1080/01902140902759290. Exp Lung Res. 2009. PMID: 19842832 Review.
Cited by
-
Tollip SNP rs5743899 modulates human airway epithelial responses to rhinovirus infection.Clin Exp Allergy. 2016 Dec;46(12):1549-1563. doi: 10.1111/cea.12793. Epub 2016 Sep 21. Clin Exp Allergy. 2016. PMID: 27513438 Free PMC article.
-
Fine Particulate Matter (PM2.5) Promotes CD146 Expression in Alveolar Epithelial Cells and Cryptococcus neoformans Pulmonary Infection.Front Microbiol. 2021 Jan 18;11:525976. doi: 10.3389/fmicb.2020.525976. eCollection 2020. Front Microbiol. 2021. PMID: 33537006 Free PMC article.
-
Proteomics analysis reveals a Th17-prone cell population in presymptomatic graft-versus-host disease.JCI Insight. 2016 May 5;1(6):e86660. doi: 10.1172/jci.insight.86660. JCI Insight. 2016. PMID: 27195312 Free PMC article.
-
Lung Pericytes in Pulmonary Vascular Physiology and Pathophysiology.Compr Physiol. 2021 Jun 30;11(3):2227-2247. doi: 10.1002/cphy.c200027. Compr Physiol. 2021. PMID: 34190345 Free PMC article.
-
CD146 Associates with Gp130 to Control a Macrophage Pro-inflammatory Program That Regulates the Metabolic Response to Obesity.Adv Sci (Weinh). 2022 May;9(13):e2103719. doi: 10.1002/advs.202103719. Epub 2022 Mar 8. Adv Sci (Weinh). 2022. PMID: 35258174 Free PMC article.
References
-
- Celli B.R., Barnes P.J. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29:1224–1238. [Erratum appeared in Eur Respir J 2007, 30:401] - PubMed
-
- Anzueto A., Sethi S., Martinez F.J. Exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4:554–564. - PubMed
-
- Rabe K.F., Hurd S., Anzueto A., Barnes P.J., Buist S.A., Calverley P., Fukuchi Y., Jenkins C., Rodriguez-Roisin R., van Weel C., Zielinski J. Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. - PubMed
-
- Dalcin Pde T., Perin C. [Management of acute asthma in adults in the emergency room: current evidence]. Portuguese. Rev Assoc Med Bras. 2009;55:82–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

