The highly conserved layer-3 component of the HIV-1 gp120 inner domain is critical for CD4-required conformational transitions
- PMID: 23255784
- PMCID: PMC3571356
- DOI: 10.1128/JVI.03104-12
The highly conserved layer-3 component of the HIV-1 gp120 inner domain is critical for CD4-required conformational transitions
Abstract
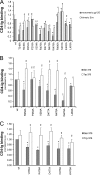
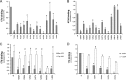
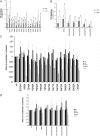
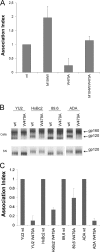
The trimeric envelope glycoprotein (Env) of human immunodeficiency virus type 1 (HIV-1) mediates virus entry into host cells. CD4 engagement with the gp120 exterior envelope glycoprotein subunit represents the first step during HIV-1 entry. CD4-induced conformational changes in the gp120 inner domain involve three potentially flexible topological layers (layers 1, 2, and 3). Structural rearrangements between layer 1 and layer 2 have been shown to facilitate the transition of the envelope glycoprotein trimer from the unliganded to the CD4-bound state and to stabilize gp120-CD4 interaction. However, our understanding of CD4-induced conformational changes in the gp120 inner domain remains incomplete. Here, we report that a highly conserved element of the gp120 inner domain, layer 3, plays a pivot-like role in these allosteric changes. In the unliganded state, layer 3 modulates the association of gp120 with the Env trimer, probably by influencing the relationship of the gp120 inner and outer domains. Importantly, layer 3 governs the efficiency of the initial gp120 interaction with CD4, a function that can also be fulfilled by filling the Phe43 cavity. This work defines the functional importance of layer 3 and completes a picture detailing the role of the gp120 inner domain in CD4-induced conformational transitions in the HIV-1 Env trimer.
Figures



Similar articles
-
Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions.Mol Cell. 2010 Mar 12;37(5):656-67. doi: 10.1016/j.molcel.2010.02.012. Mol Cell. 2010. PMID: 20227370 Free PMC article.
-
Lineage-Specific Differences between the gp120 Inner Domain Layer 3 of Human Immunodeficiency Virus and That of Simian Immunodeficiency Virus.J Virol. 2016 Oct 28;90(22):10065-10073. doi: 10.1128/JVI.01215-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27535053 Free PMC article.
-
Characterization of Human Immunodeficiency Virus (HIV-1) Envelope Glycoprotein Variants Selected for Resistance to a CD4-Mimetic Compound.J Virol. 2022 Sep 14;96(17):e0063622. doi: 10.1128/jvi.00636-22. Epub 2022 Aug 18. J Virol. 2022. PMID: 35980207 Free PMC article.
-
Structure-based design, synthesis and validation of CD4-mimetic small molecule inhibitors of HIV-1 entry: conversion of a viral entry agonist to an antagonist.Acc Chem Res. 2014 Apr 15;47(4):1228-37. doi: 10.1021/ar4002735. Epub 2014 Feb 6. Acc Chem Res. 2014. PMID: 24502450 Free PMC article. Review.
-
Quaternary Interaction of the HIV-1 Envelope Trimer with CD4 and Neutralizing Antibodies.Viruses. 2021 Jul 20;13(7):1405. doi: 10.3390/v13071405. Viruses. 2021. PMID: 34372611 Free PMC article. Review.
Cited by
-
The HIV-1 gp120 major variable regions modulate cold inactivation.J Virol. 2013 Apr;87(7):4103-11. doi: 10.1128/JVI.03124-12. Epub 2013 Jan 23. J Virol. 2013. PMID: 23345516 Free PMC article.
-
Regulation of epitope exposure in the gp41 membrane-proximal external region through interactions at the apex of HIV-1 Env.PLoS Pathog. 2022 May 18;18(5):e1010531. doi: 10.1371/journal.ppat.1010531. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35584191 Free PMC article.
-
Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity.J Virol. 2014 Mar;88(5):2633-44. doi: 10.1128/JVI.03230-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352444 Free PMC article.
-
Distinct conformations of the HIV-1 V3 loop crown are targetable for broad neutralization.Nat Commun. 2021 Nov 18;12(1):6705. doi: 10.1038/s41467-021-27075-0. Nat Commun. 2021. PMID: 34795280 Free PMC article.
-
Short communication: Anti-HIV-1 envelope immunoglobulin Gs in blood and cervicovaginal samples of Beninese commercial sex workers.AIDS Res Hum Retroviruses. 2014 Nov;30(11):1145-9. doi: 10.1089/aid.2014.0163. AIDS Res Hum Retroviruses. 2014. PMID: 25354025 Free PMC article.
References
-
- Allan JS, Coligan JE, Barin F, McLane MF, Sodroski JG, Rosen CA, Haseltine WA, Lee TH, Essex M. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091–1094 - PubMed
-
- Robey WG, Safai B, Oroszlan S, Arthur LO, Gonda MA, Gallo RC, Fischinger PJ. 1985. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science 228:593–595 - PubMed
-
- Yang X, Mahony E, Holm GH, Kassa A, Sodroski J. 2003. Role of the gp120 inner domain beta-sandwich in the interaction between the human immunodeficiency virus envelope glycoprotein subunits. Virology 313:117–125 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

