Mouse macrophage innate immune response to Chikungunya virus infection
- PMID: 23253140
- PMCID: PMC3577478
- DOI: 10.1186/1743-422X-9-313
Mouse macrophage innate immune response to Chikungunya virus infection
Abstract
Background: Infection with Chikungunya alphavirus (CHIKV) can cause severe arthralgia and chronic arthritis in humans with persistence of the virus in perivascular macrophages of the synovial membrane by mechanisms largely ill-characterized.
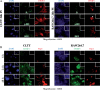
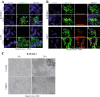
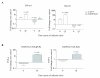
Findings: We herein analysed the innate immune response (cytokine and programmed cell death) of RAW264.7 mouse macrophages following CHIKV infection. We found that the infection was restrained to a small percentage of cells and was not associated with a robust type I IFN innate immune response (IFN-α4 and ISG56). TNF-α, IL-6 and GM-CSF expression were upregulated while IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-10 or IL-17 expression could not be evidenced prior to and after CHIKV exposure. Although CHIKV is known to drive apoptosis in many cell types, we found no canonical signs of programmed cell death (cleaved caspase-3, -9) in infected RAW264.7 cells.
Conclusion: These data argue for the capacity of CHIKV to infect and drive a specific innate immune response in RAW264.7 macrophage cell which seems to be polarized to assist viral persistence through the control of apoptosis and IFN signalling.
Figures


Similar articles
-
Regulation of Viral Replication, Apoptosis and Pro-Inflammatory Responses by 17-AAG during Chikungunya Virus Infection in Macrophages.Viruses. 2017 Jan 6;9(1):3. doi: 10.3390/v9010003. Viruses. 2017. PMID: 28067803 Free PMC article.
-
Deciphering the differential response of two human fibroblast cell lines following Chikungunya virus infection.Virol J. 2012 Sep 20;9:213. doi: 10.1186/1743-422X-9-213. Virol J. 2012. PMID: 22992396 Free PMC article.
-
Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response.J Immunol. 2010 May 15;184(10):5903-13. doi: 10.4049/jimmunol.0904181. Epub 2010 Apr 19. J Immunol. 2010. PMID: 20404274
-
Chikungunya Virus-Induced Arthritis: Role of Host and Viral Factors in the Pathogenesis.Viral Immunol. 2017 Dec;30(10):691-702. doi: 10.1089/vim.2017.0052. Epub 2017 Sep 14. Viral Immunol. 2017. PMID: 28910194 Review.
-
Immune-Mediated Protection and Pathogenesis of Chikungunya Virus.J Immunol. 2016 Dec 1;197(11):4210-4218. doi: 10.4049/jimmunol.1601426. J Immunol. 2016. PMID: 27864552 Free PMC article. Review.
Cited by
-
Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses.Biomolecules. 2021 May 18;11(5):753. doi: 10.3390/biom11050753. Biomolecules. 2021. PMID: 34069869 Free PMC article.
-
A comparison of Chikungunya virus infection, progression, and cytokine profiles in human PMA-differentiated U937 and murine RAW264.7 monocyte derived macrophages.PLoS One. 2020 Mar 12;15(3):e0230328. doi: 10.1371/journal.pone.0230328. eCollection 2020. PLoS One. 2020. PMID: 32163514 Free PMC article.
-
Cellular and Molecular Immune Response to Chikungunya Virus Infection.Front Cell Infect Microbiol. 2018 Oct 10;8:345. doi: 10.3389/fcimb.2018.00345. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30364124 Free PMC article. Review.
-
Molecular mechanisms involved in the pathogenesis of alphavirus-induced arthritis.Biomed Res Int. 2013;2013:973516. doi: 10.1155/2013/973516. Epub 2013 Aug 28. Biomed Res Int. 2013. PMID: 24069610 Free PMC article. Review.
-
TLR4 is one of the receptors for Chikungunya virus envelope protein E2 and regulates virus induced pro-inflammatory responses in host macrophages.Front Immunol. 2023 Apr 20;14:1139808. doi: 10.3389/fimmu.2023.1139808. eCollection 2023. Front Immunol. 2023. PMID: 37153546 Free PMC article.
References
-
- Mathiot CC, Grimaud G, Garry P, Bouquety JC, Mada A, Daguisy AM, Georges AJ. An outbreak of human Semliki Forest virus infections in Central African Republic. Am J Trop Med Hyg. 1990;42:386–393. - PubMed
-
- Jaffar-Bandjee MC, Das T, Hoarau JJ, Krejbich Trotot P, Denizot M, Ribera A, Roques P, Gasque P. Chikungunya virus takes centre stage in virally induced arthritis: possible cellular and molecular mechanisms to pathogenesis. Microbes and infection / Institut Pasteur. 2009;11:1206–1218. doi: 10.1016/j.micinf.2009.10.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

