Advances in the pathogenesis of Alzheimer's disease: focusing on tau-mediated neurodegeneration
- PMID: 23241453
- PMCID: PMC3598890
- DOI: 10.1186/2047-9158-1-24
Advances in the pathogenesis of Alzheimer's disease: focusing on tau-mediated neurodegeneration
Abstract
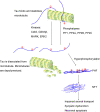
In addition to senile plaques and cerebral amyloid angiopathy, the hyperphosphorylation of tau protein and formation of intraneuronal neurofibrillary tangles (NFTs) represents another neuropathological hallmark in AD brain. Tau is a microtubule-associated protein and localizes predominantly in the axons of neurons with the primary function in maintaining microtubules stability. When the balance between tau phosphorylation and dephosphorylation is changed in favor of the former, tau is hyperphosphorylated and the level of the free tau fractions elevated. The hyperphosphorylation of tau protein and formation of NFTs represent a characteristic neuropathological feature in AD brain. We have discussed the role of Aβ in AD in our previous review, this review focused on the recent advances in tau-mediated AD pathology, mainly including tau hyperphosphorylation, propagation of tau pathology and the relationship between tau and Aβ.
Figures
Similar articles
-
[Alzheimer disease: cellular and molecular aspects].Bull Mem Acad R Med Belg. 2005;160(10-12):445-9; discussion 450-1. Bull Mem Acad R Med Belg. 2005. PMID: 16768248 French.
-
Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2005 Jan;2(1):3-18. doi: 10.2174/1567205052772713. Curr Alzheimer Res. 2005. PMID: 15977985 Review.
-
Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer's disease.J Alzheimers Dis. 2009;16(1):15-27. doi: 10.3233/JAD-2009-0960. J Alzheimers Dis. 2009. PMID: 19158417 Review.
-
Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles.J Neural Transm Suppl. 1998;53:169-80. doi: 10.1007/978-3-7091-6467-9_15. J Neural Transm Suppl. 1998. PMID: 9700655 Review.
-
Pathological Tau From Alzheimer's Brain Induces Site-Specific Hyperphosphorylation and SDS- and Reducing Agent-Resistant Aggregation of Tau in vivo.Front Aging Neurosci. 2019 Mar 5;11:34. doi: 10.3389/fnagi.2019.00034. eCollection 2019. Front Aging Neurosci. 2019. PMID: 30890929 Free PMC article.
Cited by
-
MicroRNA-146a suppresses ROCK1 allowing hyperphosphorylation of tau in Alzheimer's disease.Sci Rep. 2016 May 25;6:26697. doi: 10.1038/srep26697. Sci Rep. 2016. PMID: 27221467 Free PMC article.
-
Axonal degeneration in the anterior insular cortex is associated with Alzheimer's co-pathology in Parkinson's disease and dementia with Lewy bodies.Transl Neurodegener. 2022 Dec 7;11(1):52. doi: 10.1186/s40035-022-00325-x. Transl Neurodegener. 2022. PMID: 36474289 Free PMC article.
-
Insights into Disease-Associated Tau Impact on Mitochondria.Int J Mol Sci. 2020 Sep 1;21(17):6344. doi: 10.3390/ijms21176344. Int J Mol Sci. 2020. PMID: 32882957 Free PMC article. Review.
-
Stability properties of neuronal microtubules.Cytoskeleton (Hoboken). 2016 Sep;73(9):442-60. doi: 10.1002/cm.21286. Cytoskeleton (Hoboken). 2016. PMID: 26887570 Free PMC article. Review.
-
Polysaccharides from Basella alba Protect Post-Mitotic Neurons against Cell Cycle Re-Entry and Apoptosis Induced by the Amyloid-Beta Peptide by Blocking Sonic Hedgehog Expression.Int J Mol Sci. 2024 Jul 3;25(13):7316. doi: 10.3390/ijms25137316. Int J Mol Sci. 2024. PMID: 39000427 Free PMC article.
References
LinkOut - more resources
Full Text Sources

