Human cytomegalovirus pUL29/28 and pUL38 repression of p53-regulated p21CIP1 and caspase 1 promoters during infection
- PMID: 23236067
- PMCID: PMC3571358
- DOI: 10.1128/JVI.01926-12
Human cytomegalovirus pUL29/28 and pUL38 repression of p53-regulated p21CIP1 and caspase 1 promoters during infection
Abstract
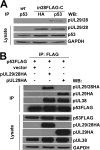
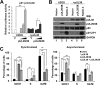
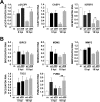
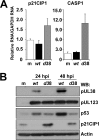
During infection by human cytomegalovirus (HCMV), the tumor suppressor protein p53, which promotes efficient viral gene expression, is stabilized. However, the expression of numerous p53-responsive cellular genes is not upregulated. The molecular mechanism used to manipulate the transcriptional activity of p53 during infection remains unclear. The HCMV proteins IE1, IE2, pUL44, and pUL84 likely contribute to the regulation of p53. In this study, we used a discovery-based approach to identify the protein targets of the HCMV protein pUL29/28 during infection. Previous studies have demonstrated that pUL29/28 regulates viral gene expression by interacting with the chromatin remodeling complex NuRD. Here, we observed that pUL29/28 also associates with p53, an additional deacetylase complex, and several HCMV proteins, including pUL38. We confirmed the interaction between p53 and pUL29/28 in both the presence and absence of infection. HCMV pUL29/28 with pUL38 altered the activity of the 53-regulatable p21CIP1 promoter. During infection, pUL29/28 and pUL38 contributed to the inhibition of p21CIP1 as well as caspase 1 expression. The expression of several other p53-regulating genes was not altered. Infection using a UL29-deficient virus resulted in increased p53 binding and histone H3 acetylation at the responsive promoters. Furthermore, expression of pUL29/28 and its interacting partner pUL38 contributed to an increase in the steady-state protein levels of p53. This study identified two additional HCMV proteins, pUL29/28 and pUL38, which participate in the complex regulation of p53 transcriptional activity during infection.
Figures

Similar articles
-
Human cytomegalovirus UL29/28 protein interacts with components of the NuRD complex which promote accumulation of immediate-early RNA.PLoS Pathog. 2010 Jun 24;6(6):e1000965. doi: 10.1371/journal.ppat.1000965. PLoS Pathog. 2010. PMID: 20585571 Free PMC article.
-
Human Cytomegalovirus Protein pUL38 Prevents Premature Cell Death by Binding to Ubiquitin-Specific Protease 24 and Regulating Iron Metabolism.J Virol. 2018 Jun 13;92(13):e00191-18. doi: 10.1128/JVI.00191-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29695420 Free PMC article.
-
Tuberous Sclerosis Complex Protein 2-Independent Activation of mTORC1 by Human Cytomegalovirus pUL38.J Virol. 2015 Aug;89(15):7625-35. doi: 10.1128/JVI.01027-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972538 Free PMC article.
-
Chromatin-mediated regulation of cytomegalovirus gene expression.Virus Res. 2011 May;157(2):134-43. doi: 10.1016/j.virusres.2010.09.019. Epub 2010 Sep 25. Virus Res. 2011. PMID: 20875471 Free PMC article. Review.
-
Human cytomegalovirus riding the cell cycle.Med Microbiol Immunol. 2015 Jun;204(3):409-19. doi: 10.1007/s00430-015-0396-z. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776080 Review.
Cited by
-
Can thymosin beta 10 function both as a non-invasive biomarker and chemotherapeutic target in human colorectal cancer?Transl Oncol. 2024 Aug;46:102026. doi: 10.1016/j.tranon.2024.102026. Epub 2024 Jun 7. Transl Oncol. 2024. PMID: 38850800 Free PMC article.
-
Interaction network of proteins associated with human cytomegalovirus IE2-p86 protein during infection: a proteomic analysis.PLoS One. 2013 Dec 16;8(12):e81583. doi: 10.1371/journal.pone.0081583. eCollection 2013. PLoS One. 2013. PMID: 24358118 Free PMC article.
-
Antagonistic Relationship between Human Cytomegalovirus pUL27 and pUL97 Activities during Infection.J Virol. 2015 Oct;89(20):10230-46. doi: 10.1128/JVI.00986-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223645 Free PMC article.
-
DNA repair mechanisms and human cytomegalovirus (HCMV) infection.Folia Microbiol (Praha). 2015 May;60(3):199-209. doi: 10.1007/s12223-014-0359-6. Epub 2014 Nov 1. Folia Microbiol (Praha). 2015. PMID: 25366712 Free PMC article. Review.
-
Emerging Mechanisms of G1/S Cell Cycle Control by Human and Mouse Cytomegaloviruses.mBio. 2021 Dec 21;12(6):e0293421. doi: 10.1128/mBio.02934-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903047 Free PMC article. Review.
References
-
- Mocarski E, Shenk T, Pass RF. 2007. Cytomegaloviruses, p 2702–2772 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA
-
- Cannon MJ. 2009. Congenital cytomegalovirus (CMV) epidemiology and awareness. J. Clin. Virol. 46(Suppl 4):S6–S10 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

