Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells
- PMID: 23235743
- PMCID: PMC3612556
- DOI: 10.1038/mi.2012.123
Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells
Abstract
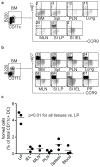
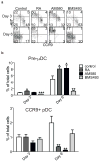
The vitamin A metabolite retinoic acid (RA) regulates intestinal immune responses through immunomodulatory actions on intestinal dendritic cells (DCs) and lymphocytes. Here, we show that RA also controls the generation of gut-tropic migratory DC precursors, referred to as pre-mucosal DCs (pre-μDCs). Pre-μDCs express the gut trafficking receptor α4β7 and home preferentially to the intestines. They develop in the bone marrow (BM), can differentiate into CCR9⁺ plasmacytoid DCs as well as conventional DCs (cDCs), but preferentially give rise to CD103⁺ intestinal cDCs. Generation of pre-μDCs in vivo in the BM or in vitro is regulated by RA and RA receptor α (RARα) signaling. The frequency of pre-μDCs is reduced in vitamin A-deficient animals and in animals treated with RAR inhibitors. The results define a novel vitamin A-dependent, RA-regulated developmental sequence for DCs and identify a targeted precursor for CD103⁺ cDCs in the gut.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells.Mucosal Immunol. 2011 Jul;4(4):438-47. doi: 10.1038/mi.2010.91. Epub 2011 Feb 2. Mucosal Immunol. 2011. PMID: 21289617 Free PMC article.
-
Generation and transcriptional programming of intestinal dendritic cells: essential role of retinoic acid.Mucosal Immunol. 2016 Jan;9(1):183-93. doi: 10.1038/mi.2015.50. Epub 2015 Jul 1. Mucosal Immunol. 2016. PMID: 26129652 Free PMC article.
-
Butyrate and retinoic acid imprint mucosal-like dendritic cell development synergistically from bone marrow cells.Clin Exp Immunol. 2017 Sep;189(3):290-297. doi: 10.1111/cei.12990. Epub 2017 Jun 13. Clin Exp Immunol. 2017. PMID: 28542882 Free PMC article.
-
Development and functional specialization of CD103+ dendritic cells.Immunol Rev. 2010 Mar;234(1):268-81. doi: 10.1111/j.0105-2896.2009.00874.x. Immunol Rev. 2010. PMID: 20193025 Review.
-
How vitamin A metabolizing dendritic cells are generated in the gut mucosa.Trends Immunol. 2012 Jan;33(1):42-8. doi: 10.1016/j.it.2011.10.001. Epub 2011 Nov 11. Trends Immunol. 2012. PMID: 22079120 Review.
Cited by
-
Vedolizumab for inflammatory bowel disease: Changing the game, or more of the same?United European Gastroenterol J. 2014 Oct;2(5):333-44. doi: 10.1177/2050640614550672. United European Gastroenterol J. 2014. PMID: 25360311 Free PMC article. Review.
-
A novel in vitro model of the small intestinal epithelium in co-culture with 'gut-like' dendritic cells.Discov Immunol. 2023 Oct 7;2(1):kyad018. doi: 10.1093/discim/kyad018. eCollection 2023. Discov Immunol. 2023. PMID: 38567056 Free PMC article.
-
Dietary Glucose Consumption Promotes RALDH Activity in Small Intestinal CD103+CD11b+ Dendritic Cells.Front Immunol. 2020 Aug 11;11:1897. doi: 10.3389/fimmu.2020.01897. eCollection 2020. Front Immunol. 2020. PMID: 32849649 Free PMC article.
-
Host and microbial factors in regulation of T cells in the intestine.Front Immunol. 2013 Jun 10;4:141. doi: 10.3389/fimmu.2013.00141. eCollection 2013. Front Immunol. 2013. PMID: 23772228 Free PMC article.
-
RARα supports the development of Langerhans cells and langerin-expressing conventional dendritic cells.Nat Commun. 2018 Sep 25;9(1):3896. doi: 10.1038/s41467-018-06341-8. Nat Commun. 2018. PMID: 30254197 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007290/AI/NIAID NIH HHS/United States
- AI047822/AI/NIAID NIH HHS/United States
- DK084647/DK/NIDDK NIH HHS/United States
- DK085426/DK/NIDDK NIH HHS/United States
- AI007290/AI/NIAID NIH HHS/United States
- R01 AI093981/AI/NIAID NIH HHS/United States
- R37 AI047822/AI/NIAID NIH HHS/United States
- AI093981/AI/NIAID NIH HHS/United States
- P30 DK056339/DK/NIDDK NIH HHS/United States
- 5 T32 AI07290/AI/NIAID NIH HHS/United States
- R01 DK084647/DK/NIDDK NIH HHS/United States
- R21 AI047822/AI/NIAID NIH HHS/United States
- R03 DK085426/DK/NIDDK NIH HHS/United States
- R01 AI047822/AI/NIAID NIH HHS/United States
- DK056339/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

