Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial
- PMID: 23233715
- PMCID: PMC3732014
- DOI: 10.1200/JCO.2012.44.4711
Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial
Abstract
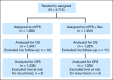
Purpose: The National Surgical Adjuvant Breast and Bowel Project trial C-08 was designed to investigate the safety and efficacy of adding bevacizumab to fluorouracil, leucovorin, and oxaliplatin (FOLFOX6) for the adjuvant treatment of patients with stage 2-3 colon cancer. Our report summarizes the primary and secondary end points of disease-free and overall survival, respectively, with 5 years median follow-up time.
Patients and methods: Patients received modified FOLFOX6 once every 2 weeks for a 6-month period (control group) or modified FOLFOX6 for 6 months plus bevacizumab (5 mg/kg) once every 2 weeks for a 12-month period (experimental group). The primary end point of the study was disease-free survival (DFS) and overall survival (OS) was a secondary end point.
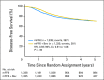
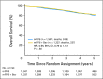
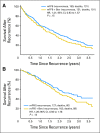
Results: Of 2,673 analyzed patients, demographic factors were well-balanced by treatment. With a median follow-up of 5 years, the addition of bevacizumab to mFOLFOX6 did not result in an overall significant increase in DFS (hazard ratio [HR], 0.93; 95% CI, 0.81 to 1.08; P = .35). Exploratory analyses found that the effect of bevacizumab on DFS was different before and after a 1.25-year landmark (time-by-treatment interaction P value <.0001). The secondary end point of OS was no different between the two study arms for all patients (HR, 0.95; 95% CI, 0.79 to 1.13; P = .56) and for those with stage 3 disease (HR, 1.0; 95% CI, 0.83 to 1.21; P = .99).
Conclusion: Bevacizumab for 1 year with modified FOLFOX6 does not significantly prolong DFS or OS in stage 2-3 colon cancer. We observed no evidence of a detrimental effect of exposure to bevacizumab. A transient effect on disease-free survival was observed during bevacizumab exposure in the study's experimental arm.
Trial registration: ClinicalTrials.gov NCT00096278.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08.J Clin Oncol. 2011 Jan 1;29(1):11-6. doi: 10.1200/JCO.2010.30.0855. Epub 2010 Oct 12. J Clin Oncol. 2011. PMID: 20940184 Free PMC article. Clinical Trial.
-
Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer.J Clin Oncol. 2009 Jul 10;27(20):3385-90. doi: 10.1200/JCO.2009.21.9220. Epub 2009 May 4. J Clin Oncol. 2009. PMID: 19414665 Free PMC article. Clinical Trial.
-
Association of Bevacizumab Plus Oxaliplatin-Based Chemotherapy With Disease-Free Survival and Overall Survival in Patients With Stage II Colon Cancer: A Secondary Analysis of the AVANT Trial.JAMA Netw Open. 2020 Oct 1;3(10):e2020425. doi: 10.1001/jamanetworkopen.2020.20425. JAMA Netw Open. 2020. PMID: 33074326 Free PMC article. Clinical Trial.
-
Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial.Lancet Oncol. 2012 Dec;13(12):1225-33. doi: 10.1016/S1470-2045(12)70509-0. Epub 2012 Nov 16. Lancet Oncol. 2012. PMID: 23168362 Clinical Trial.
-
Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study.J Clin Oncol. 2015 Dec 10;33(35):4176-87. doi: 10.1200/JCO.2015.63.4238. Epub 2015 Nov 2. J Clin Oncol. 2015. PMID: 26527776 Clinical Trial.
Cited by
-
Targeting HIBCH to reprogram valine metabolism for the treatment of colorectal cancer.Cell Death Dis. 2019 Aug 13;10(8):618. doi: 10.1038/s41419-019-1832-6. Cell Death Dis. 2019. PMID: 31409769 Free PMC article.
-
Kidney cancer: Rest ASSUREd, much can be learned from adjuvant studies in renal cancer.Nat Rev Nephrol. 2016 Jun;12(6):317-8. doi: 10.1038/nrneph.2016.58. Epub 2016 Apr 25. Nat Rev Nephrol. 2016. PMID: 27108841 Free PMC article.
-
Prognostic value of microvessel density in stage II and III colon cancer patients: a retrospective cohort study.BMC Gastroenterol. 2019 Aug 16;19(1):146. doi: 10.1186/s12876-019-1063-4. BMC Gastroenterol. 2019. PMID: 31420015 Free PMC article.
-
Perioperative Chemotherapy for Liver Metastasis of Colorectal Cancer: Lessons Learned and Future Perspectives.Curr Treat Options Oncol. 2022 Sep;23(9):1320-1337. doi: 10.1007/s11864-022-01008-5. Epub 2022 Aug 18. Curr Treat Options Oncol. 2022. PMID: 35980520 Review.
-
Effect of Adjuvant Chemotherapy on Elderly Colorectal Cancer Patients: Lack of Evidence.Gastrointest Tumors. 2017 Sep;4(1-2):11-19. doi: 10.1159/000479318. Epub 2017 Aug 31. Gastrointest Tumors. 2017. PMID: 29071260 Free PMC article. Review.
References
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med . 2004;350:2343–2351. - PubMed
-
- Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol . 2007;25:2198–2204. - PubMed
-
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol . 2007;25:1539–1544. - PubMed
-
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. [Errata: J Clin Oncol 26:3110, 2008 and J Clin Oncol 27:653, 2009] - PubMed
-
- Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: The BRiTE observational cohort study. Oncologist . 2009;14:862–870. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

