Homeostatic tissue responses in skin biopsies from NOMID patients with constitutive overproduction of IL-1β
- PMID: 23226210
- PMCID: PMC3511496
- DOI: 10.1371/journal.pone.0049408
Homeostatic tissue responses in skin biopsies from NOMID patients with constitutive overproduction of IL-1β
Abstract
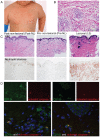
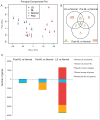
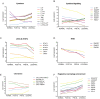
The autoinflammatory disorder, Neonatal-onset Multisystem Inflammatory Disease (NOMID) is the most severe phenotype of disorders caused by mutations in CIAS1 that result in increased production and secretion of active IL-1β. NOMID patients present with systemic and organ-specific inflammation of the skin, central nervous system and bone, and respond dramatically to treatment with IL-1 blocking agents. We compared the cellular infiltrates and transcriptome of skin biopsies from patients with NOMID (n = 14) before treatment (lesional (LS) and non-lesional (pre-NL) skin) and after treatment (post-NL) with the IL-1 blocker anakinra (recombinant IL-1 receptor antagonist, Kineret®, Swedish Orphan Biovitrum AB, SOBI), to normal skin (n = 5) to assess tissue responses in the context of untreated and treated disease. Abundant neutrophils distinguish LS skin from pre-NL and post-NL skin. CD11c(+) dermal dendritic cells and CD163(+) macrophages expressed activated caspase-1 and are a likely source of cutaneous IL-1 production. Treatment with anakinra led to the disappearance of neutrophils, but CD3(+) T cells and HLA-DR(+) cells remained elevated. Among the upregulated genes IL-6, IL-8, TNF, IL-17A, CCL20, and the neutrophil defensins DEFA1 and DEFA3 were differentially regulated in LS tissues (compared to normal skin). Important significantly downregulated pathways in LS skin included IL-1R/TLR signaling, type I and II cytokine receptor signaling, mitochondrial dysfunction, and antigen presentation. The differential expression and regulation of microRNAs and pathways involved in post-transcriptional modification were suggestive of epigenetic modification in the chronically inflamed tissue. Overall, the dysregulated genes and pathways suggest extensive "adaptive" mechanisms to control inflammation and maintain tissue homeostasis, likely triggered by chronic IL-1 release in the skin of patients with NOMID.
Conflict of interest statement
Figures

Similar articles
-
Current status of understanding the pathogenesis and management of patients with NOMID/CINCA.Curr Rheumatol Rep. 2011 Apr;13(2):123-31. doi: 10.1007/s11926-011-0165-y. Curr Rheumatol Rep. 2011. PMID: 21538043 Free PMC article. Review.
-
[Cutaneous neutrophils infiltrates. Case 8. CINCA/NOMID syndrome].Ann Pathol. 2011 Jun;31(3):203-7. doi: 10.1016/j.annpat.2011.05.005. Epub 2011 Jun 12. Ann Pathol. 2011. PMID: 21737003 French. No abstract available.
-
Identification of Interleukin-1β-Producing Monocytes That Are Susceptible to Pyronecrotic Cell Death in Patients With Neonatal-Onset Multisystem Inflammatory Disease.Arthritis Rheumatol. 2015 Dec;67(12):3286-97. doi: 10.1002/art.39307. Arthritis Rheumatol. 2015. PMID: 26245468 Free PMC article.
-
"Mutation negative" familial cold autoinflammatory syndrome (FCAS) in an 8-year-old boy: clinical course and functional studies.Rheumatol Int. 2012 Sep;32(9):2629-36. doi: 10.1007/s00296-011-2019-3. Epub 2011 Jul 22. Rheumatol Int. 2012. PMID: 21833523
-
Current treatment recommendations and considerations for cryopyrin-associated periodic syndrome.Expert Rev Clin Immunol. 2015;11(10):1083-92. doi: 10.1586/1744666X.2015.1077702. Epub 2015 Aug 27. Expert Rev Clin Immunol. 2015. PMID: 26312542 Review.
Cited by
-
The Double-Stranded RNA Analog, Poly(I:C), Triggers Distinct Transcriptomic Shifts in Keratinocyte Subsets.J Invest Dermatol. 2022 Oct;142(10):2820-2823.e1. doi: 10.1016/j.jid.2022.03.015. Epub 2022 Apr 5. J Invest Dermatol. 2022. PMID: 35395221 Free PMC article. No abstract available.
-
Pathogenic NLRP3 mutants form constitutively active inflammasomes resulting in immune-metabolic limitation of IL-1β production.Nat Commun. 2024 Feb 6;15(1):1096. doi: 10.1038/s41467-024-44990-0. Nat Commun. 2024. PMID: 38321014 Free PMC article.
-
Current and future advances in genetic testing in systemic autoinflammatory diseases.Rheumatology (Oxford). 2019 Nov 1;58(Suppl 6):vi44-vi55. doi: 10.1093/rheumatology/kez294. Rheumatology (Oxford). 2019. PMID: 31769854 Free PMC article. Review.
-
Cellular Mechanisms of Psoriasis Pathogenesis: A Systemic Review.Clin Cosmet Investig Dermatol. 2023 Sep 14;16:2503-2515. doi: 10.2147/CCID.S420850. eCollection 2023. Clin Cosmet Investig Dermatol. 2023. PMID: 37727872 Free PMC article. Review.
-
Treating inflammation by blocking interleukin-1 in humans.Semin Immunol. 2013 Dec 15;25(6):469-84. doi: 10.1016/j.smim.2013.10.008. Epub 2013 Nov 23. Semin Immunol. 2013. PMID: 24275598 Free PMC article. Review.
References
-
- Saito M, Nishikomori R, Kambe N, Fujisawa A, Tanizaki H, et al. (2008) Disease-associated CIAS1 mutations induce monocyte death, revealing low-level mosaicism in mutation-negative cryopyrin-associated periodic syndrome patients. Blood 111: 2132–2141. - PubMed
-
- Caroli F, Pontillo A, D'Osualdo A, Travan L, Ceccherini I, et al. (2007) Clinical and genetic characterization of Italian patients affected by CINCA syndrome. Rheumatology (Oxford) 46: 473–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

