Studying calcium-triggered vesicle fusion in a single vesicle-vesicle content and lipid-mixing system
- PMID: 23222454
- PMCID: PMC3566647
- DOI: 10.1038/nprot.2012.134
Studying calcium-triggered vesicle fusion in a single vesicle-vesicle content and lipid-mixing system
Abstract
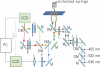
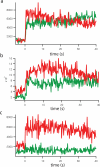
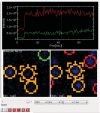
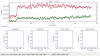
This protocol describes a single vesicle-vesicle microscopy system to study Ca(2+)-triggered vesicle fusion. Donor vesicles contain reconstituted synaptobrevin and synaptotagmin-1. Acceptor vesicles contain reconstituted syntaxin and synaptosomal-associated protein 25 (SNAP-25), and they are tethered to a PEG-coated glass surface. Donor vesicles are mixed with the tethered acceptor vesicles and incubated for several minutes at a zero-Ca(2+) concentration, resulting in a collection of single interacting vesicle pairs. The donor vesicles also contain two spectrally distinct fluorophores that allow simultaneous monitoring of temporal changes of the content and membrane. Upon Ca(2+) injection into the sample chamber, our system therefore differentiates between hemifusion and complete fusion of interacting vesicle pairs and determines the temporal sequence of these events on a sub-100-millisecond time scale. Other factors such as complexin can be easily added. Our system is unique in that it monitors both content and lipid mixing and starts from a metastable state of interacting vesicle pairs before Ca(2+) injection.
Figures





Similar articles
-
Munc18a does not alter fusion rates mediated by neuronal SNAREs, synaptotagmin, and complexin.J Biol Chem. 2015 Apr 17;290(16):10518-34. doi: 10.1074/jbc.M114.630772. Epub 2015 Feb 25. J Biol Chem. 2015. PMID: 25716318 Free PMC article.
-
In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release.Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):E304-13. doi: 10.1073/pnas.1107900108. Epub 2011 Jun 24. Proc Natl Acad Sci U S A. 2011. PMID: 21705659 Free PMC article.
-
Complexin inhibits spontaneous release and synchronizes Ca2+-triggered synaptic vesicle fusion by distinct mechanisms.Elife. 2014 Aug 13;3:e03756. doi: 10.7554/eLife.03756. Elife. 2014. PMID: 25122624 Free PMC article.
-
Ca2+-Triggered Synaptic Vesicle Fusion Initiated by Release of Inhibition.Trends Cell Biol. 2018 Aug;28(8):631-645. doi: 10.1016/j.tcb.2018.03.004. Epub 2018 Apr 26. Trends Cell Biol. 2018. PMID: 29706534 Free PMC article. Review.
-
Conflicting views on the membrane fusion machinery and the fusion pore.Annu Rev Cell Dev Biol. 2009;25:513-37. doi: 10.1146/annurev.cellbio.24.110707.175239. Annu Rev Cell Dev Biol. 2009. PMID: 19575641 Review.
Cited by
-
Munc18a does not alter fusion rates mediated by neuronal SNAREs, synaptotagmin, and complexin.J Biol Chem. 2015 Apr 17;290(16):10518-34. doi: 10.1074/jbc.M114.630772. Epub 2015 Feb 25. J Biol Chem. 2015. PMID: 25716318 Free PMC article.
-
pH Dependence of Zika Membrane Fusion Kinetics Reveals an Off-Pathway State.ACS Cent Sci. 2018 Nov 28;4(11):1503-1510. doi: 10.1021/acscentsci.8b00494. Epub 2018 Oct 12. ACS Cent Sci. 2018. PMID: 30555902 Free PMC article.
-
Molecular Mechanisms of Synaptic Vesicle Priming by Munc13 and Munc18.Neuron. 2017 Aug 2;95(3):591-607.e10. doi: 10.1016/j.neuron.2017.07.004. Neuron. 2017. PMID: 28772123 Free PMC article.
-
Towards reconstitution of membrane fusion mediated by SNAREs and other synaptic proteins.Crit Rev Biochem Mol Biol. 2015;50(3):231-41. doi: 10.3109/10409238.2015.1023252. Epub 2015 Mar 19. Crit Rev Biochem Mol Biol. 2015. PMID: 25788028 Free PMC article. Review.
-
Preincubation of t-SNAREs with Complexin I Increases Content-Mixing Efficiency.Biochemistry. 2016 Jul 5;55(26):3667-73. doi: 10.1021/acs.biochem.6b00114. Epub 2016 Jun 24. Biochemistry. 2016. PMID: 27286417 Free PMC article.
References
-
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
-
- Bean AJ, Zhang X, Hokfelt T. Peptide secretion: what do we know? FASEB J. 1994;8(9):630–638. - PubMed
-
- Voets T, Neher E, Moser T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron. 1999;23(3):607–615. - PubMed
-
- Lindau M, Gomperts BD. Techniques and concepts in exocytosis: focus on mast cells. Biochim Biophys Acta. 1991;1071(4):429–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

