Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein
- PMID: 23209422
- PMCID: PMC3510253
- DOI: 10.1371/journal.ppat.1003059
Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein
Abstract
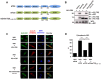
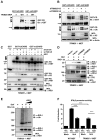
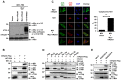
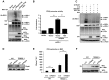
Influenza A viruses can adapt to new host species, leading to the emergence of novel pathogenic strains. There is evidence that highly pathogenic viruses encode for non-structural 1 (NS1) proteins that are more efficient in suppressing the host immune response. The NS1 protein inhibits type-I interferon (IFN) production partly by blocking the TRIM25 ubiquitin E3 ligase-mediated Lys63-linked ubiquitination of the viral RNA sensor RIG-I, required for its optimal downstream signaling. In order to understand possible mechanisms of viral adaptation and host tropism, we examined the ability of NS1 encoded by human (Cal04), avian (HK156), swine (SwTx98) and mouse-adapted (PR8) influenza viruses to interact with TRIM25 orthologues from mammalian and avian species. Using co-immunoprecipitation assays we show that human TRIM25 binds to all tested NS1 proteins, whereas the chicken TRIM25 ortholog binds preferentially to the NS1 from the avian virus. Strikingly, none of the NS1 proteins were able to bind mouse TRIM25. Since NS1 can inhibit IFN production in mouse, we tested the impact of TRIM25 and NS1 on RIG-I ubiquitination in mouse cells. While NS1 efficiently suppressed human TRIM25-dependent ubiquitination of RIG-I 2CARD, NS1 inhibited the ubiquitination of full-length mouse RIG-I in a mouse TRIM25-independent manner. Therefore, we tested if the ubiquitin E3 ligase Riplet, which has also been shown to ubiquitinate RIG-I, interacts with NS1. We found that NS1 binds mouse Riplet and inhibits its activity to induce IFN-β in murine cells. Furthermore, NS1 proteins of human but not swine or avian viruses were able to interact with human Riplet, thereby suppressing RIG-I ubiquitination. In conclusion, our results indicate that influenza NS1 protein targets TRIM25 and Riplet ubiquitin E3 ligases in a species-specific manner for the inhibition of RIG-I ubiquitination and antiviral IFN production.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I.Cell Host Microbe. 2009 May 8;5(5):439-49. doi: 10.1016/j.chom.2009.04.006. Cell Host Microbe. 2009. PMID: 19454348 Free PMC article.
-
A distinct role of Riplet-mediated K63-Linked polyubiquitination of the RIG-I repressor domain in human antiviral innate immune responses.PLoS Pathog. 2013;9(8):e1003533. doi: 10.1371/journal.ppat.1003533. Epub 2013 Aug 8. PLoS Pathog. 2013. PMID: 23950712 Free PMC article.
-
Avian Influenza NS1 Proteins Inhibit Human, but Not Duck, RIG-I Ubiquitination and Interferon Signaling.J Virol. 2022 Sep 28;96(18):e0077622. doi: 10.1128/jvi.00776-22. Epub 2022 Sep 7. J Virol. 2022. PMID: 36069546 Free PMC article.
-
The influenza virus NS1 protein as a therapeutic target.Antiviral Res. 2013 Sep;99(3):409-16. doi: 10.1016/j.antiviral.2013.06.005. Epub 2013 Jun 21. Antiviral Res. 2013. PMID: 23796981 Free PMC article. Review.
-
Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I.J Biochem. 2012 Jan;151(1):5-11. doi: 10.1093/jb/mvr111. Epub 2011 Sep 2. J Biochem. 2012. PMID: 21890623 Review.
Cited by
-
Evolutionary features of a prolific subtype of avian influenza A virus in European waterfowl.Virus Evol. 2022 Aug 27;8(2):veac074. doi: 10.1093/ve/veac074. eCollection 2022. Virus Evol. 2022. PMID: 36128050 Free PMC article.
-
TRIM Proteins and Their Roles in the Influenza Virus Life Cycle.Microorganisms. 2020 Sep 16;8(9):1424. doi: 10.3390/microorganisms8091424. Microorganisms. 2020. PMID: 32947942 Free PMC article. Review.
-
TREX (transcription/export)-NP complex exerts a dual effect on regulating polymerase activity and replication of influenza A virus.PLoS Pathog. 2022 Sep 9;18(9):e1010835. doi: 10.1371/journal.ppat.1010835. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36084138 Free PMC article.
-
The Cellular DExD/H-Box RNA Helicase UAP56 Co-localizes With the Influenza A Virus NS1 Protein.Front Microbiol. 2018 Sep 12;9:2192. doi: 10.3389/fmicb.2018.02192. eCollection 2018. Front Microbiol. 2018. PMID: 30258431 Free PMC article.
-
The Molecular Mechanism of Herpes Simplex Virus 1 UL31 in Antagonizing the Activity of IFN-β.Microbiol Spectr. 2022 Feb 23;10(1):e0188321. doi: 10.1128/spectrum.01883-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196784 Free PMC article.
References
-
- Johnson NP, Mueller J (2002) Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med 76: 105–115. - PubMed
-
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, et al. (2007) The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25: 5086–5096. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

