Depletion of Bmi-1 enhances 5-fluorouracil-induced apoptosis and autophagy in hepatocellular carcinoma cells
- PMID: 23205090
- PMCID: PMC3506668
- DOI: 10.3892/ol.2012.805
Depletion of Bmi-1 enhances 5-fluorouracil-induced apoptosis and autophagy in hepatocellular carcinoma cells
Abstract
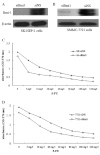
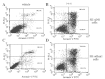
5-fluorouracil (5-FU) is one of the standard chemoradiotherapy regimens for hepatocellular carcinoma (HCC) treatment. B-cell-specific Moloney murine leukemia virus insertion site 1 (Bmi-1) has been demonstrated to regulate proliferation. Additionally, Bmi-1 overexpression has been identified in HCC cell lines and correlates with the advanced invasive stage of tumor progression and poor prognosis. In this study, we examined the effects of 5-FU treatment on cell growth in HCC cells with or without Bmi-1 depletion. The IC(50) values of 5-FU were significantly decreased to a greater extent in cells with Bmi-1 knockdown. Depletion of Bmi-1 increased sensitivity of the cells to 5-FU and increased apoptosis. Knockdown of endogenous Bmi-1 led to a substantial reduction in the levels of phospho-AKT and Bcl-2 with a concomitant increase in the levels of Bax. Additionally, 5-FU induced the conversion/turnover of microtubule-associated protein 1 light chain 3 (LC3). Knockdown of endogenous Bmi-1 led to an increase in the levels of Beclin-1 and the accumulation of LC3-II. Together, these findings reveal that Bmi-1 depletion enhanced the chemosensitivity of HCC cells by inducing apoptosis and autophagy, which is associated with the PI3K/AKT and Bcl-2/Beclin-1 pathways.
Figures

Similar articles
-
Downregulation of BMI-1 enhances 5-fluorouracil-induced apoptosis in nasopharyngeal carcinoma cells.Biochem Biophys Res Commun. 2008 Jul 4;371(3):531-5. doi: 10.1016/j.bbrc.2008.04.117. Epub 2008 Apr 29. Biochem Biophys Res Commun. 2008. PMID: 18452707
-
RBFOX3 Regulates the Chemosensitivity of Cancer Cells to 5-Fluorouracil via the PI3K/AKT, EMT and Cytochrome-C/Caspase Pathways.Cell Physiol Biochem. 2018;46(4):1365-1380. doi: 10.1159/000489153. Epub 2018 Apr 18. Cell Physiol Biochem. 2018. PMID: 29689552
-
Opsin3 sensitizes hepatocellular carcinoma cells to 5-fluorouracil treatment by regulating the apoptotic pathway.Cancer Lett. 2012 Jul 1;320(1):96-103. doi: 10.1016/j.canlet.2012.01.035. Epub 2012 Feb 4. Cancer Lett. 2012. PMID: 22313545
-
Knockdown of PRDX2 sensitizes colon cancer cells to 5-FU by suppressing the PI3K/AKT signaling pathway.Biosci Rep. 2017 May 11;37(3):BSR20160447. doi: 10.1042/BSR20160447. Print 2017 Jun 30. Biosci Rep. 2017. PMID: 28432271 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
Camellia nitidissima Chi Extract Potentiates the Sensitivity of Gastric Cancer Cells to Paclitaxel via the Induction of Autophagy and Apoptosis.Onco Targets Ther. 2019 Dec 11;12:10811-10825. doi: 10.2147/OTT.S220453. eCollection 2019. Onco Targets Ther. 2019. PMID: 31853183 Free PMC article.
-
Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy.Cells. 2021 Nov 17;10(11):3213. doi: 10.3390/cells10113213. Cells. 2021. PMID: 34831435 Free PMC article.
-
The novel BMI-1 inhibitor PTC596 downregulates MCL-1 and induces p53-independent mitochondrial apoptosis in acute myeloid leukemia progenitor cells.Blood Cancer J. 2017 Feb 17;7(2):e527. doi: 10.1038/bcj.2017.8. Blood Cancer J. 2017. PMID: 28211885 Free PMC article.
-
BMI-1, a promising therapeutic target for human cancer.Oncol Lett. 2015 Aug;10(2):583-588. doi: 10.3892/ol.2015.3361. Epub 2015 Jun 11. Oncol Lett. 2015. PMID: 26622537 Free PMC article.
-
Blocking autophagic flux enhances matrine-induced apoptosis in human hepatoma cells.Int J Mol Sci. 2013 Nov 25;14(12):23212-30. doi: 10.3390/ijms141223212. Int J Mol Sci. 2013. PMID: 24287900 Free PMC article.
References
-
- Nagano H. Treatment of advanced hepatocellular carcinoma: intraarterial infusion chemotherapy combined with interferon. Oncology. 2010;78(Suppl 1):142–147. - PubMed
-
- Boulin M, Guiu S, Chauffert B, Aho S, Cercueil JP, Ghiringhelli F, Krause D, Fagnoni P, Hillon P, Bedenne L, Guiu B. Screening of anticancer drugs for chemoembolization of hepatocellular carcinoma. Anticancer Drugs. 2011;22:741–748. - PubMed
-
- Haupt Y, Alexander WS, Barri G, Klinken SP, Adams JM. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell. 1991;65:753–763. - PubMed
-
- van Lohuizen M, Verbeek S, Scheijen B, Wientjens E, van der Gulden H, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991;65:737–752. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
