Size-dependent mechanism of cargo sorting during lysosome-phagosome fusion is controlled by Rab34
- PMID: 23197834
- PMCID: PMC3528610
- DOI: 10.1073/pnas.1206811109
Size-dependent mechanism of cargo sorting during lysosome-phagosome fusion is controlled by Rab34
Abstract
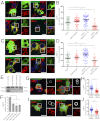
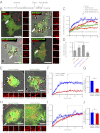
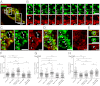
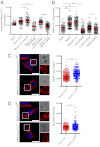
Phagosome maturation is an essential part of the innate and adaptive immune response. Although it is well established that several Ras-related proteins in brain (Rab) proteins become associated to phagosomes, little is known about how these phagosomal Rab proteins influence phagosome maturation. Here, we show a specific role for Rab34 and mammalian uncoordinated 13-2 (Munc13-2) in phagolysosome biogenesis and cargo delivery. Rab34 knockdown impaired the fusion of phagosomes with late endosomes/lysosomes and high levels of active Rab34 promoted this process. We demonstrate that Rab34 enhances phagosome maturation independently of Rab7 and coordinates phagolysosome biogenesis through size-selective transfer of late endosomal/lysosomal cargo into phagosomes. More importantly, we show that Rab34 mediates phagosome maturation through the recruitment of the protein Munc13-2. Finally, we report that the alternative maturation pathway controlled by Rab34 is critical for mycobacterial killing because Rab34 silencing resulted in mycobacterial survival, and Rab34 expression led to mycobacterial killing. Altogether, our studies uncover Rab34/Munc13-2 as a critical part of an alternative Rab7-independent phagosome maturation machinery and lysosome-mediated killing of mycobacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest.EMBO J. 2006 Nov 15;25(22):5250-9. doi: 10.1038/sj.emboj.7601407. Epub 2006 Nov 2. EMBO J. 2006. PMID: 17082769 Free PMC article.
-
Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes.Traffic. 2011 Apr;12(4):407-20. doi: 10.1111/j.1600-0854.2011.01165.x. Epub 2011 Feb 21. Traffic. 2011. PMID: 21255211
-
Spatial distribution of phagolysosomes is independent of the regulation of lysosome position by Rab34.Int J Biochem Cell Biol. 2013 Sep;45(9):2057-65. doi: 10.1016/j.biocel.2013.07.003. Epub 2013 Jul 17. Int J Biochem Cell Biol. 2013. PMID: 23871933
-
[Alterations in recruitment and activation of Rab proteins during mycobacterial infection].Biomedica. 2010 Apr-Jun;30(2):283-308. Biomedica. 2010. PMID: 20890576 Review. Spanish.
-
Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking.Electrophoresis. 1997 Dec;18(14):2542-7. doi: 10.1002/elps.1150181409. Electrophoresis. 1997. PMID: 9527483 Review.
Cited by
-
Phagocytosis of antibody-opsonized tumor cells leads to the formation of a discrete vacuolar compartment in macrophages.Traffic. 2018 Apr;19(4):273-284. doi: 10.1111/tra.12552. Traffic. 2018. PMID: 29437282 Free PMC article.
-
The Role of Rab GTPases in the development of genetic and malignant diseases.Mol Cell Biochem. 2024 Feb;479(2):255-281. doi: 10.1007/s11010-023-04727-x. Epub 2023 Apr 15. Mol Cell Biochem. 2024. PMID: 37060515 Review.
-
Innate immune receptors for cross-presentation: The expanding role of NLRs.Mol Immunol. 2019 Sep;113:6-10. doi: 10.1016/j.molimm.2017.11.028. Epub 2017 Nov 29. Mol Immunol. 2019. PMID: 29198621 Free PMC article. Review.
-
Interferon-γ-inducible Rab20 regulates endosomal morphology and EGFR degradation in macrophages.Mol Biol Cell. 2015 Sep 1;26(17):3061-70. doi: 10.1091/mbc.E14-11-1547. Epub 2015 Jul 8. Mol Biol Cell. 2015. PMID: 26157167 Free PMC article.
-
Pathogenic RAB34 variants impair primary cilium assembly and cause a novel oral-facial-digital syndrome.Hum Mol Genet. 2023 Sep 5;32(18):2822-2831. doi: 10.1093/hmg/ddad109. Hum Mol Genet. 2023. PMID: 37384395 Free PMC article.
References
-
- Stuart LM, Ezekowitz RA. Phagocytosis: Elegant complexity. Immunity. 2005;22(5):539–550. - PubMed
-
- Flannagan RS, Cosío G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7(5):355–366. - PubMed
-
- Jutras I, Desjardins M. Phagocytosis: At the crossroads of innate and adaptive immunity. Annu Rev Cell Dev Biol. 2005;21:511–527. - PubMed
-
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

