Impaired therapeutic capacity of autologous stem cells in a model of type 2 diabetes
- PMID: 23197759
- PMCID: PMC3659680
- DOI: 10.5966/sctm.2012-0031
Impaired therapeutic capacity of autologous stem cells in a model of type 2 diabetes
Abstract
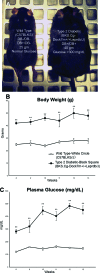
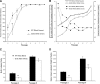
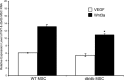
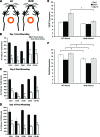
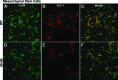
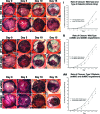
Endogenous stem cells in the bone marrow respond to environmental cues and contribute to tissue maintenance and repair. In type 2 diabetes, a multifaceted metabolic disease characterized by insulin resistance and hyperglycemia, major complications are seen in multiple organ systems. To evaluate the effects of this disease on the endogenous stem cell population, we used a type 2 diabetic mouse model (db/db), which recapitulates these diabetic phenotypes. Bone marrow-derived mesenchymal stem cells (MSCs) from db/db mice were characterized in vitro using flow cytometric cell population analysis, differentiation, gene expression, and proliferation assays. Diabetic MSCs were evaluated for their therapeutic potential in vivo using an excisional splint wound model in both nondiabetic wild-type and diabetic mice. Diabetic animals possessed fewer MSCs, which were proliferation and survival impaired in vitro. Examination of the recruitment response of stem and progenitor cells after wounding revealed that significantly fewer endogenous MSCs homed to the site of injury in diabetic subjects. Although direct engraftment of healthy MSCs accelerated wound closure in both healthy and diabetic subjects, diabetic MSC engraftment produced limited improvement in the diabetic subjects and could not produce the same therapeutic outcomes as in their nondiabetic counterparts in vivo. Our data reveal stem cell impairment as a major complication of type 2 diabetes in mice and suggest that the disease may stably alter endogenous MSCs. These results have implications for the efficiency of autologous therapies in diabetic patients and identify endogenous MSCs as a potential therapeutic target.
Figures
Similar articles
-
Cxcr6-Based Mesenchymal Stem Cell Gene Therapy Potentiates Skin Regeneration in Murine Diabetic Wounds.Mol Ther. 2020 May 6;28(5):1314-1326. doi: 10.1016/j.ymthe.2020.02.014. Epub 2020 Feb 14. Mol Ther. 2020. PMID: 32112713 Free PMC article.
-
The effect of estrogen on diabetic wound healing is mediated through increasing the function of various bone marrow-derived progenitor cells.J Vasc Surg. 2018 Dec;68(6S):127S-135S. doi: 10.1016/j.jvs.2018.04.069. Epub 2018 Jul 29. J Vasc Surg. 2018. PMID: 30064832
-
Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice.J Am Heart Assoc. 2012 Dec;1(6):e002238. doi: 10.1161/JAHA.112.002238. Epub 2012 Dec 19. J Am Heart Assoc. 2012. PMID: 23316315 Free PMC article.
-
Impact of Diabetes Mellitus on Human Mesenchymal Stromal Cell Biology and Functionality: Implications for Autologous Transplantation.Stem Cell Rev Rep. 2019 Apr;15(2):194-217. doi: 10.1007/s12015-018-9869-y. Stem Cell Rev Rep. 2019. PMID: 30680660 Review.
-
Mesenchymal stem cells as a treatment for peripheral arterial disease: current status and potential impact of type II diabetes on their therapeutic efficacy.Stem Cell Rev Rep. 2013 Jun;9(3):360-72. doi: 10.1007/s12015-013-9433-8. Stem Cell Rev Rep. 2013. PMID: 23475434 Free PMC article. Review.
Cited by
-
Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine.Stem Cells Dev. 2013 Jun 15;22(12):1752-62. doi: 10.1089/scd.2012.0688. Epub 2013 Mar 15. Stem Cells Dev. 2013. PMID: 23363023 Free PMC article.
-
Alteration of immunoregulatory genes expression in mesenchymal stromal cells upon priming with B18R as an interferon binding protein.Iran J Basic Med Sci. 2023 Feb;26(2):241-247. doi: 10.22038/IJBMS.2022.67353.14771. Iran J Basic Med Sci. 2023. PMID: 36742146 Free PMC article.
-
Systematic review and meta-analysis of mouse models of diabetes-associated ulcers.BMJ Open Diabetes Res Care. 2020 May;8(1):e000982. doi: 10.1136/bmjdrc-2019-000982. BMJ Open Diabetes Res Care. 2020. PMID: 32467222 Free PMC article. Review.
-
Senescence in Adipose-Derived Stem Cells: Biological Mechanisms and Therapeutic Challenges.Int J Mol Sci. 2024 Aug 1;25(15):8390. doi: 10.3390/ijms25158390. Int J Mol Sci. 2024. PMID: 39125960 Free PMC article. Review.
-
Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: stem cell transplantation methods that enhance stemness.Stem Cell Res Ther. 2015 Nov 5;6:215. doi: 10.1186/s13287-015-0217-8. Stem Cell Res Ther. 2015. PMID: 26541973 Free PMC article. Review.
References
-
- Bild DE, Selby JV, Sinnock P, et al. Lower-extremity amputation in people with diabetes: Epidemiology and prevention. Diabetes Care. 1989;12:24–31. - PubMed
-
- Trousdale RK, Jacobs S, Simhaee DA, et al. Wound closure and metabolic parameter variability in a db/db mouse model for diabetic ulcers. J Surg Res. 2009;151:100–107. - PubMed
-
- Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. - PubMed
-
- Kuroda Y, Kitada M, Wakao S, et al. Bone marrow mesenchymal cells: How do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp (Warsz) 2011;59:369–378. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

