S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury
- PMID: 23190274
- PMCID: PMC3684103
- DOI: 10.1089/neu.2012.2579
S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury
Abstract
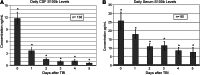
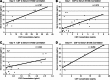
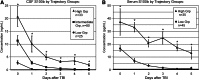
As an astrocytic protein specific to the central nervous system, S100b is a potentially useful marker in outcome prediction after traumatic brain injury (TBI). Some studies have questioned the validity of S100b, citing the extracerebral origins of the protein as reducing the specificity of the marker. This study evaluated S100b as a prognostic biomarker in adult subjects with severe TBI (sTBI) by comparing outcomes with S100b temporal profiles generated from both cerebrospinal fluid (CSF) (n = 138 subjects) and serum (n = 80 subjects) samples across a 6-day time course. Long-bone fracture, Injury Severity Score (ISS), and isolated head injury status were variables used to assess extracerebral sources of S100b in serum. After TBI, CSF and serum S100b levels were increased over healthy controls across the first 6 days post-TBI (p ≤ 0.005 and p ≤ 0.031). Though CSF and serum levels were highly correlated during early time points post-TBI, this association diminished over time. Bivariate analysis showed that subjects who had temporal CSF profiles with higher S100b concentrations had higher acute mortality (p < 0.001) and worse Glasgow Outcome Scale (GOS; p = 0.002) and Disability Rating Scale (DRS) scores (p = 0.039) 6 months post-injury. Possibly as a result of extracerebral sources of S100b in serum, as represented by high ISS scores (p = 0.032), temporal serum profiles were associated with acute mortality (p = 0.015). High CSF S100b levels were observed in women (p = 0.022) and older subjects (p = 0.004). Multivariate logistic regression confirmed CSF S100b profiles in predicting GOS and DRS and showed mean and peak serum S100b as acute mortality predictors after sTBI.
Figures
Similar articles
-
Early CSF and Serum S100B Concentrations for Outcome Prediction in Traumatic Brain Injury and Subarachnoid Hemorrhage.Clin Neurol Neurosurg. 2016 Jun;145:79-83. doi: 10.1016/j.clineuro.2016.04.005. Epub 2016 Apr 8. Clin Neurol Neurosurg. 2016. PMID: 27101088
-
Predicting outcome after severe traumatic brain injury using the serum S100B biomarker: results using a single (24h) time-point.Resuscitation. 2009 Mar;80(3):341-5. doi: 10.1016/j.resuscitation.2008.11.021. Epub 2009 Jan 15. Resuscitation. 2009. PMID: 19150161
-
The prognostic significance of the serum biomarker heart-fatty acidic binding protein in comparison with s100b in severe traumatic brain injury.J Neurotrauma. 2013 Oct 1;30(19):1631-7. doi: 10.1089/neu.2012.2791. J Neurotrauma. 2013. PMID: 23590685
-
Association between S100B Levels and Long-Term Outcome after Aneurysmal Subarachnoid Hemorrhage: Systematic Review and Pooled Analysis.PLoS One. 2016 Mar 23;11(3):e0151853. doi: 10.1371/journal.pone.0151853. eCollection 2016. PLoS One. 2016. PMID: 27007976 Free PMC article. Review.
-
Reliability of S100B in predicting severity of central nervous system injury.Neurocrit Care. 2007;6(2):121-38. doi: 10.1007/s12028-007-0008-x. Neurocrit Care. 2007. PMID: 17522796 Review.
Cited by
-
Blood biomarkers for brain injury: What are we measuring?Neurosci Biobehav Rev. 2016 Sep;68:460-473. doi: 10.1016/j.neubiorev.2016.05.009. Epub 2016 May 12. Neurosci Biobehav Rev. 2016. PMID: 27181909 Free PMC article. Review.
-
Biomarkers for the clinical differential diagnosis in traumatic brain injury--a systematic review.CNS Neurosci Ther. 2013 Aug;19(8):556-65. doi: 10.1111/cns.12127. Epub 2013 May 27. CNS Neurosci Ther. 2013. PMID: 23710877 Free PMC article. Review.
-
Raman Spectroscopy Spectral Fingerprints of Biomarkers of Traumatic Brain Injury.Cells. 2023 Nov 8;12(22):2589. doi: 10.3390/cells12222589. Cells. 2023. PMID: 37998324 Free PMC article.
-
S100B Serum Level as a Mortality Predictor for Traumatic Brain Injury: A Meta-Analysis.Open Access Maced J Med Sci. 2018 Nov 15;6(11):2239-2244. doi: 10.3889/oamjms.2018.432. eCollection 2018 Nov 25. Open Access Maced J Med Sci. 2018. PMID: 30559895 Free PMC article. Review.
-
Status of precision medicine approaches to traumatic brain injury.Neural Regen Res. 2022 Oct;17(10):2166-2171. doi: 10.4103/1673-5374.335824. Neural Regen Res. 2022. PMID: 35259824 Free PMC article. Review.
References
-
- Steyerberg E.W. Mushkudiani N. Perel P. Butcher I. Lu J. McHugh G.S. Murray G.D. Marmarou A. Roberts I. Habbema J.D. Maas A.I. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:1251–1261. - PMC - PubMed
-
- MRC CRASH Trial Collaborators. Perel P. Arango M. Clayton T. Edwards P. Komolafe E. Poccock S. Roberts I. Shakur H. Steyerberg E. Yutthakasemsunt S. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–429. - PMC - PubMed
-
- Hukkelhoven C.W. Rampen A.J. Maas A.I. Farace E. Habbema J.D. Marmarou A. Marshall L.F. Murray G.D. Steyerberg E.W. Some prognostic models for traumatic brain injury were not valid. J. Clin. Epidemiol. 2006;59:132–143. - PubMed
-
- Maas A.I. Marmarou A. Murray G.D. Teasdale S.G. Steyerberg E.W. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J. Neurotrauma. 2007;24:232–238. - PubMed
-
- Berger R.P. The use of serum biomarkers to predict outcome after traumatic brain injury in adults and children. J. Head Trauma Rehabil. 2006;21:315–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

