Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation
- PMID: 23186290
- PMCID: PMC3525721
- DOI: 10.1021/bi301441e
Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation
Abstract
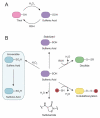
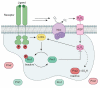
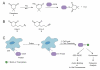
Epidermal growth factor receptor (EGFR) exemplifies the family of receptor tyrosine kinases that mediate numerous cellular processes, including growth, proliferation, and differentiation. Moreover, gene amplification and EGFR mutations have been identified in a number of human malignancies, making this receptor an important target for the development of anticancer drugs. In addition to ligand-dependent activation and concomitant tyrosine phosphorylation, EGFR stimulation results in the localized generation of H(2)O(2) by NADPH-dependent oxidases. In turn, H(2)O(2) functions as a secondary messenger to regulate intracellular signaling cascades, largely through the modification of specific cysteine residues within redox-sensitive protein targets, including Cys797 in the EGFR active site. In this review, we highlight recent advances in our understanding of the mechanisms that underlie redox regulation of EGFR signaling and how these discoveries may form the basis for the development of new therapeutic strategies for targeting this and other H(2)O(2)-modulated pathways.
Figures

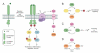
Similar articles
-
The NADPH Oxidases DUOX1 and NOX2 Play Distinct Roles in Redox Regulation of Epidermal Growth Factor Receptor Signaling.J Biol Chem. 2016 Oct 28;291(44):23282-23293. doi: 10.1074/jbc.M116.749028. Epub 2016 Sep 20. J Biol Chem. 2016. PMID: 27650496 Free PMC article.
-
ATP-mediated transactivation of the epidermal growth factor receptor in airway epithelial cells involves DUOX1-dependent oxidation of Src and ADAM17.PLoS One. 2013;8(1):e54391. doi: 10.1371/journal.pone.0054391. Epub 2013 Jan 18. PLoS One. 2013. PMID: 23349873 Free PMC article.
-
Adenosine triphosphate regulates NADPH oxidase activity leading to hydrogen peroxide production and COX-2/PGE2 expression in A549 cells.Am J Physiol Lung Cell Mol Physiol. 2012 Sep;303(5):L401-12. doi: 10.1152/ajplung.00090.2012. Epub 2012 Jul 6. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22773695
-
Redox-dependent regulation of epidermal growth factor receptor signaling.Redox Biol. 2016 Aug;8:24-7. doi: 10.1016/j.redox.2015.12.002. Epub 2015 Dec 8. Redox Biol. 2016. PMID: 26722841 Free PMC article. Review.
-
Chemical approaches to discovery and study of sources and targets of hydrogen peroxide redox signaling through NADPH oxidase proteins.Annu Rev Biochem. 2015;84:765-90. doi: 10.1146/annurev-biochem-060614-034018. Annu Rev Biochem. 2015. PMID: 26034893 Free PMC article. Review.
Cited by
-
Renal FGF23 signaling depends on redox protein Memo1 and promotes orthovanadate-sensitive protein phosphotyrosyl phosphatase activity.J Cell Commun Signal. 2023 Sep;17(3):705-722. doi: 10.1007/s12079-022-00710-1. Epub 2022 Nov 25. J Cell Commun Signal. 2023. PMID: 36434320 Free PMC article.
-
The redox biochemistry of protein sulfenylation and sulfinylation.J Biol Chem. 2013 Sep 13;288(37):26480-8. doi: 10.1074/jbc.R113.467738. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861405 Free PMC article. Review.
-
Covalent inhibition of protein tyrosine phosphatases.Mol Biosyst. 2017 Jun 27;13(7):1257-1279. doi: 10.1039/c7mb00151g. Mol Biosyst. 2017. PMID: 28534914 Free PMC article. Review.
-
Synthesis of benzopentathiepin analogs and their evaluation as inhibitors of the phosphatase STEP.Bioorg Med Chem Lett. 2015 Mar 1;25(5):1044-6. doi: 10.1016/j.bmcl.2015.01.020. Epub 2015 Jan 20. Bioorg Med Chem Lett. 2015. PMID: 25666825 Free PMC article.
-
Hydrogen sulfide targets EGFR Cys797/Cys798 residues to induce Na(+)/K(+)-ATPase endocytosis and inhibition in renal tubular epithelial cells and increase sodium excretion in chronic salt-loaded rats.Antioxid Redox Signal. 2014 Nov 20;21(15):2061-82. doi: 10.1089/ars.2013.5304. Epub 2014 May 8. Antioxid Redox Signal. 2014. PMID: 24684506 Free PMC article.
References
-
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. - PubMed
-
- Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. - PubMed
-
- Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 1962;237:1555–1562. - PubMed
-
- Cohen S, Carpenter G, King L., Jr. Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J. Biol. Chem. 1980;255:4834–4842. - PubMed
-
- Carpenter G, King L, Jr., Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978;276:409–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

