GSK3beta-mediated Drp1 phosphorylation induced elongated mitochondrial morphology against oxidative stress
- PMID: 23185298
- PMCID: PMC3502545
- DOI: 10.1371/journal.pone.0049112
GSK3beta-mediated Drp1 phosphorylation induced elongated mitochondrial morphology against oxidative stress
Abstract
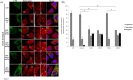
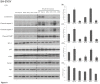
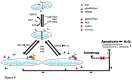
Multiple phosphorylation sites of Drp1 have been characterized for their functional importance. However, the functional consequence of GSK3beta-mediated phosphorylation of Drp1 remains unclear. In this report, we pinpointed 11 Serine/Threonine sites spanning from residue 634~736 of the GED domain and robustly confirmed Drp1 Ser693 as a novel GSK3beta phosphorylation site. Our results suggest that GSK3beta-mediated phosphorylation at Ser693 does cause a dramatic decrease of GTPase activity; in contrast, GSK3beta-mediated phosphorylation at Ser693 appears not to affect Drp1 inter-/intra-molecular interactions. After identifying Ser693 as a GSK3beta phosphorylation site, we also determined that K679 is crucial for GSK3beta-binding, which strongly suggests that Drp1 is a novel substrate for GSK3beta. Thereafter, we found that overexpressed S693D, but not S693A mutant, caused an elongated mitochondrial morphology which is similar to that of K38A, S637D and K679A mutants. Interestedly, using H89 and LiCl to inhibit PKA and GSK3beta signaling, respectively, it appears that a portion of the elongated mitochondria switched to a fragmented phenotype. In investigating the biofunctionality of phosphorylation sites within the GED domain, cells overexpressing Drp1 S693D and S637D, but not S693A, showed an acquired resistance to H(2)O(2)-induced mitochondrial fragmentation and ensuing apoptosis, which affected cytochrome c, capase-3, -7, and PARP, but not LC3B, Atg-5, Beclin-1 and Bcl2 expressions. These results also showed that the S693D group is more effective in protecting both non-neuronal and neuronal cells from apoptotic death than the S637D group. Altogether, our data suggest that GSK3beta-mediated phosphorylation at Ser693 of Drp1 may be associated with mitochondrial elongation via down-regulating apoptosis, but not autophagy upon H(2)O(2) insult.
Conflict of interest statement
Figures


Similar articles
-
GSKIP- and GSK3-mediated anchoring strengthens cAMP/PKA/Drp1 axis signaling in the regulation of mitochondrial elongation.Biochim Biophys Acta. 2015 Aug;1853(8):1796-807. doi: 10.1016/j.bbamcr.2015.04.013. Epub 2015 Apr 25. Biochim Biophys Acta. 2015. PMID: 25920809
-
Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis.Biochim Biophys Acta. 2016 Nov;1863(11):2820-2834. doi: 10.1016/j.bbamcr.2016.09.003. Epub 2016 Sep 4. Biochim Biophys Acta. 2016. PMID: 27599716
-
Blockage of GSK3β-mediated Drp1 phosphorylation provides neuroprotection in neuronal and mouse models of Alzheimer's disease.Neurobiol Aging. 2015 Jan;36(1):211-27. doi: 10.1016/j.neurobiolaging.2014.08.005. Epub 2014 Aug 8. Neurobiol Aging. 2015. PMID: 25192600
-
c-Abl-mediated Drp1 phosphorylation promotes oxidative stress-induced mitochondrial fragmentation and neuronal cell death.Cell Death Dis. 2017 Oct 12;8(10):e3117. doi: 10.1038/cddis.2017.524. Cell Death Dis. 2017. PMID: 29022905 Free PMC article.
-
Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology.J Biol Chem. 2007 Jul 27;282(30):21583-7. doi: 10.1074/jbc.C700083200. Epub 2007 Jun 6. J Biol Chem. 2007. PMID: 17553808
Cited by
-
Astragaloside IV Derivative (LS-102) Alleviated Myocardial Ischemia Reperfusion Injury by Inhibiting Drp1Ser616 Phosphorylation-Mediated Mitochondrial Fission.Front Pharmacol. 2020 Sep 17;11:1083. doi: 10.3389/fphar.2020.01083. eCollection 2020. Front Pharmacol. 2020. PMID: 33041784 Free PMC article.
-
Changes in Drp1 Function and Mitochondrial Morphology Are Associated with the α-Synuclein Pathology in a Transgenic Mouse Model of Parkinson's Disease.Cells. 2021 Apr 13;10(4):885. doi: 10.3390/cells10040885. Cells. 2021. PMID: 33924585 Free PMC article.
-
Mitochondrial dynamics: overview of molecular mechanisms.Essays Biochem. 2018 Jul 20;62(3):341-360. doi: 10.1042/EBC20170104. Print 2018 Jul 20. Essays Biochem. 2018. PMID: 30030364 Free PMC article. Review.
-
Mitochondrial dysfunction induces muscle atrophy during prolonged inactivity: A review of the causes and effects.Arch Biochem Biophys. 2019 Feb 15;662:49-60. doi: 10.1016/j.abb.2018.11.005. Epub 2018 Nov 16. Arch Biochem Biophys. 2019. PMID: 30452895 Free PMC article. Review.
-
TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue.Nat Commun. 2018 Jan 16;9(1):245. doi: 10.1038/s41467-017-02068-0. Nat Commun. 2018. PMID: 29339725 Free PMC article.
References
-
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789. - PubMed
-
- Jope RS, Johnson GV (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29: 95–102. - PubMed
-
- Hong YR, Chen CH, Cheng DS, Howng SL, Chow CC (1998) Human dynamin-like protein interacts with the glycogen synthase kinase 3beta. Biochem Biophys Res Commun 249: 697–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

