ATP hydrolysis enhances RNA recognition and antiviral signal transduction by the innate immune sensor, laboratory of genetics and physiology 2 (LGP2)
- PMID: 23184951
- PMCID: PMC3543043
- DOI: 10.1074/jbc.M112.424416
ATP hydrolysis enhances RNA recognition and antiviral signal transduction by the innate immune sensor, laboratory of genetics and physiology 2 (LGP2)
Abstract
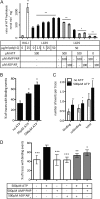
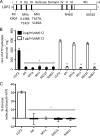
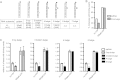
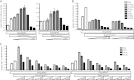
Laboratory of genetics and physiology 2 (LGP2) is a member of the RIG-I-like receptor family of cytoplasmic pattern recognition receptors that detect molecular signatures of virus infection and initiate antiviral signal transduction cascades. The ATP hydrolysis activity of LGP2 is essential for antiviral signaling, but it has been unclear how the enzymatic properties of LGP2 regulate its biological response. Quantitative analysis of the dsRNA binding and enzymatic activities of LGP2 revealed high dsRNA-independent ATP hydrolysis activity. Biochemical assays and single-molecule analysis of LGP2 and mutant variants that dissociate basal from dsRNA-stimulated ATP hydrolysis demonstrate that LGP2 utilizes basal ATP hydrolysis to enhance and diversify its RNA recognition capacity, enabling the protein to associate with intrinsically poor substrates. This property is required for LGP2 to synergize with another RIG-I-like receptor, MDA5, to potentiate IFNβ transcription in vivo during infection with encephalomyocarditis virus or transfection with poly(I:C). These results demonstrate previously unrecognized properties of LGP2 ATP hydrolysis and RNA interaction and provide a mechanistic basis for a positive regulatory role for LGP2 in antiviral signaling.
Figures
Similar articles
-
The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly.Mol Cell. 2014 Sep 4;55(5):771-81. doi: 10.1016/j.molcel.2014.07.003. Epub 2014 Aug 7. Mol Cell. 2014. PMID: 25127512 Free PMC article.
-
Viral RNA recognition by LGP2 and MDA5, and activation of signaling through step-by-step conformational changes.Nucleic Acids Res. 2020 Nov 18;48(20):11664-11674. doi: 10.1093/nar/gkaa935. Nucleic Acids Res. 2020. PMID: 33137199 Free PMC article.
-
LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling.Cytokine. 2015 Aug;74(2):198-206. doi: 10.1016/j.cyto.2015.02.010. Epub 2015 Mar 18. Cytokine. 2015. PMID: 25794939 Free PMC article. Review.
-
Differential regulation of ATP hydrolysis of RIG-I-like receptors by transactivation response RNA-binding protein.Biosci Rep. 2023 May 5;43(5):BSR20222152. doi: 10.1042/BSR20222152. Biosci Rep. 2023. PMID: 37078499 Free PMC article.
-
Activation of RIG-I-like receptor signal transduction.Crit Rev Biochem Mol Biol. 2012 Mar-Apr;47(2):194-206. doi: 10.3109/10409238.2011.630974. Epub 2011 Nov 8. Crit Rev Biochem Mol Biol. 2012. PMID: 22066529 Free PMC article. Review.
Cited by
-
Function conservation and disparities of zebrafish and human LGP2 genes in fish and mammalian cells responsive to poly(I:C).Front Immunol. 2022 Aug 17;13:985792. doi: 10.3389/fimmu.2022.985792. eCollection 2022. Front Immunol. 2022. PMID: 36059486 Free PMC article.
-
Foot-and-mouth disease virus infection inhibits LGP2 protein expression to exaggerate inflammatory response and promote viral replication.Cell Death Dis. 2017 Apr 13;8(4):e2747. doi: 10.1038/cddis.2017.170. Cell Death Dis. 2017. PMID: 28406479 Free PMC article.
-
Significant Variations in Double-Stranded RNA Levels in Cultured Skin Cells.Cells. 2024 Jan 25;13(3):226. doi: 10.3390/cells13030226. Cells. 2024. PMID: 38334619 Free PMC article.
-
Mechanisms of Non-segmented Negative Sense RNA Viral Antagonism of Host RIG-I-Like Receptors.J Mol Biol. 2019 Oct 4;431(21):4281-4289. doi: 10.1016/j.jmb.2019.06.002. Epub 2019 Jun 14. J Mol Biol. 2019. PMID: 31202887 Free PMC article. Review.
-
Negative regulators of the RIG-I-like receptor signaling pathway.Eur J Immunol. 2017 Apr;47(4):615-628. doi: 10.1002/eji.201646484. Eur J Immunol. 2017. PMID: 28295214 Free PMC article. Review.
References
-
- Yoneyama M., Fujita T. (2009) RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 227, 54–65 - PubMed
-
- Rothenfusser S., Goutagny N., DiPerna G., Gong M., Monks B. G., Schoenemeyer A., Yamamoto M., Akira S., Fitzgerald K. A. (2005) The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 175, 5260–5268 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

