Enhanced seed production under prolonged heat stress conditions in Arabidopsis thaliana plants deficient in cytosolic ascorbate peroxidase 2
- PMID: 23183257
- PMCID: PMC3528037
- DOI: 10.1093/jxb/ers335
Enhanced seed production under prolonged heat stress conditions in Arabidopsis thaliana plants deficient in cytosolic ascorbate peroxidase 2
Abstract
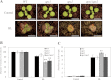
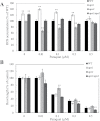
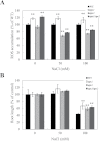
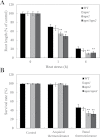
Reactive oxygen species play a key role in the response of plants to abiotic stress conditions. Their level is controlled in Arabidopsis thaliana by a large network of genes that includes the H(2)O(2)-scavenging enzymes cytosolic ascorbate peroxidase (APX) 1 and 2. Although the function of APX1 has been established under different growth conditions, genetic evidence for APX2 function, as well as for the mode of cooperation between APX1 and APX2, is very limited. This study characterized the response of Arabidopsis mutants deficient in APX1, APX2, and APX1/APX2 to heat, salinity, light, and oxidative stresses. The findings reveal that deficiency in APX2 resulted in a decreased tolerance to light stress, as well as an enhanced tolerance to salinity and oxidative stresses. Interestingly, plants lacking APX2 were more sensitive to heat stress at the seedling stage, but more tolerant to heat stress at the reproductive stage. Cooperation between APX1 and APX2 was evident during oxidative stress, but not during light, salinity, or heat stress. The findings demonstrate a role for APX2 in the response of plants to light, heat, salinity, and oxidative stresses. The finding that plants lacking APX2 produced more seeds under prolonged heat stress conditions suggests that redundant mechanisms activated in APX2-deficient plants during heat stress play a key role in the protection of reproductive tissues from heat-related damage. This finding is very important because heat-associated damage to reproductive tissues in different crops is a major cause for yield loss in agriculture production worldwide.
Figures


Similar articles
-
[The role analysis of APX gene family in the growth and developmental processes and in response to abiotic stresses in Arabidopsis thaliana].Yi Chuan. 2019 Jun 20;41(6):534-547. doi: 10.16288/j.yczz.19-026. Yi Chuan. 2019. PMID: 31257201 Chinese.
-
Cytosolic ascorbate peroxidase 1 protects organelles against oxidative stress by wounding- and jasmonate-induced H(2)O(2) in Arabidopsis plants.Biochim Biophys Acta. 2012 Dec;1820(12):1901-7. doi: 10.1016/j.bbagen.2012.08.003. Epub 2012 Aug 18. Biochim Biophys Acta. 2012. PMID: 22921811
-
Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants.Plant J. 2003 Apr;34(2):187-203. doi: 10.1046/j.1365-313x.2003.01715.x. Plant J. 2003. PMID: 12694594
-
Are diverse signalling pathways integrated in the regulation of arabidopsis antioxidant defence gene expression in response to excess excitation energy?Philos Trans R Soc Lond B Biol Sci. 2000 Oct 29;355(1402):1531-40. doi: 10.1098/rstb.2000.0713. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11128006 Free PMC article. Review.
-
Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses.Int J Mol Sci. 2015 Jun 12;16(6):13561-78. doi: 10.3390/ijms160613561. Int J Mol Sci. 2015. PMID: 26075872 Free PMC article. Review.
Cited by
-
Glutathione contributes to plant defence against parasitic cyst nematodes.Mol Plant Pathol. 2022 Jul;23(7):1048-1059. doi: 10.1111/mpp.13210. Epub 2022 Mar 29. Mol Plant Pathol. 2022. PMID: 35352464 Free PMC article.
-
ABA Is Required for Plant Acclimation to a Combination of Salt and Heat Stress.PLoS One. 2016 Jan 29;11(1):e0147625. doi: 10.1371/journal.pone.0147625. eCollection 2016. PLoS One. 2016. PMID: 26824246 Free PMC article.
-
Physiological, biochemical, and transcriptomic alterations in Castor (Ricinus communis L.) under polyethylene glycol-induced oxidative stress.BMC Plant Biol. 2024 Oct 17;24(1):973. doi: 10.1186/s12870-024-05691-4. BMC Plant Biol. 2024. PMID: 39415088 Free PMC article.
-
Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme.Redox Biol. 2023 Aug;64:102789. doi: 10.1016/j.redox.2023.102789. Epub 2023 Jun 16. Redox Biol. 2023. PMID: 37352686 Free PMC article. Review.
-
Transcriptional Profiling and Identification of Heat-Responsive Genes in Perennial Ryegrass by RNA-Sequencing.Front Plant Sci. 2017 Jun 21;8:1032. doi: 10.3389/fpls.2017.01032. eCollection 2017. Front Plant Sci. 2017. PMID: 28680431 Free PMC article.
References
-
- Bechtold U, Richard O, Zamboni A, Gapper C, Geisler M, Pogson B, Karpinski S, Mullineaux PM. 2008. Impact of chloroplastic- and extracellular-sourced ROS on high light-responsive gene expression in Arabidopsis . Journal of Experimental Botany 59, 121–133 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

