Decorin expression is important for age-related changes in tendon structure and mechanical properties
- PMID: 23178232
- PMCID: PMC3615887
- DOI: 10.1016/j.matbio.2012.11.005
Decorin expression is important for age-related changes in tendon structure and mechanical properties
Abstract
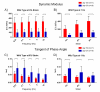
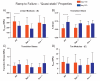
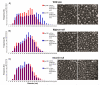
The aging population is at an increased risk of tendon injury and tendinopathy. Elucidating the molecular basis of tendon aging is crucial to understanding the age-related changes in structure and function in this vulnerable tissue. In this study, the structural and functional features of tendon aging are investigated. In addition, the roles of decorin and biglycan in the aging process were analyzed using transgenic mice at both mature and aged time points. Our hypothesis is that the increase in tendon injuries in the aging population is the result of altered structural properties that reduce the biomechanical function of the tendon and consequently increase susceptibility to injury. Decorin and biglycan are important regulators of tendon structure and therefore, we further hypothesized that decreased function in aged tendons is partly the result of altered decorin and biglycan expression. Biomechanical analyses of mature (day 150) and aged (day 570) patellar tendons revealed deteriorating viscoelastic properties with age. Histology and polarized light microscopy demonstrated decreased cellularity, alterations in tenocyte shape, and reduced collagen fiber alignment in the aged tendons. Ultrastructural analysis of fibril diameter distributions indicated an altered distribution in aged tendons with an increase of large diameter fibrils. Aged wild type tendons maintained expression of decorin which was associated with the structural and functional changes seen in aged tendons. Aged patellar tendons exhibited altered and generally inferior properties across multiple assays. However, decorin-null tendons exhibited significantly decreased effects of aging compared to the other genotypes. The amelioration of the functional deficits seen in the absence of decorin in aged tendons was associated with altered tendon fibril structure. Fibril diameter distributions in the decorin-null aged tendons were comparable to those observed in the mature wild type tendon with the absence of the subpopulation containing large diameter fibrils. Collectively, our findings provide evidence for age-dependent alterations in tendon architecture and functional activity, and further show that lack of stromal decorin attenuates these changes.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

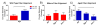

Similar articles
-
Decorin and/or biglycan knockdown in aged mouse patellar tendon impacts fibril morphology, scar area, and mechanical properties.J Orthop Res. 2024 Nov;42(11):2400-2413. doi: 10.1002/jor.25931. Epub 2024 Jul 5. J Orthop Res. 2024. PMID: 38967120
-
The injury response of aged tendons in the absence of biglycan and decorin.Matrix Biol. 2014 Apr;35:232-8. doi: 10.1016/j.matbio.2013.10.008. Epub 2013 Oct 21. Matrix Biol. 2014. PMID: 24157578 Free PMC article.
-
Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons.Matrix Biol. 2017 Dec;64:81-93. doi: 10.1016/j.matbio.2017.08.004. Epub 2017 Sep 5. Matrix Biol. 2017. PMID: 28882761 Free PMC article.
-
Proteoglycans and diseases of soft tissues.Adv Exp Med Biol. 2014;802:49-58. doi: 10.1007/978-94-007-7893-1_4. Adv Exp Med Biol. 2014. PMID: 24443020 Review.
-
Small Leucine-Rich Proteoglycans in Tendon Wound Healing.Adv Wound Care (New Rochelle). 2022 Apr;11(4):202-214. doi: 10.1089/wound.2021.0069. Epub 2021 Dec 31. Adv Wound Care (New Rochelle). 2022. PMID: 34978952 Review.
Cited by
-
Collagen V deficiency during murine tendon healing results in distinct healing outcomes based on knockdown severity.J Biomech. 2022 Nov;144:111315. doi: 10.1016/j.jbiomech.2022.111315. Epub 2022 Sep 30. J Biomech. 2022. PMID: 36201909 Free PMC article.
-
Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage.J Orthop Res. 2015 Jun;33(6):904-10. doi: 10.1002/jor.22875. Epub 2015 Apr 27. J Orthop Res. 2015. PMID: 25773654 Free PMC article.
-
Decorin and/or biglycan knockdown in aged mouse patellar tendon impacts fibril morphology, scar area, and mechanical properties.J Orthop Res. 2024 Nov;42(11):2400-2413. doi: 10.1002/jor.25931. Epub 2024 Jul 5. J Orthop Res. 2024. PMID: 38967120
-
Age and Intrinsic Fitness Affect the Female Rotator Cuff Tendon Tissue.Biomedicines. 2022 Feb 21;10(2):509. doi: 10.3390/biomedicines10020509. Biomedicines. 2022. PMID: 35203717 Free PMC article.
-
Exploring new therapeutic potentials of curcumin against post-surgical adhesion bands.BMC Complement Med Ther. 2023 Jan 31;23(1):27. doi: 10.1186/s12906-022-03808-6. BMC Complement Med Ther. 2023. PMID: 36721147 Free PMC article.
References
-
- Bailey AJ, Shimokomaki MS. Age related changes in the reducible cross-links of collagen. FEBS Lett. 1971;16(2):86–88. - PubMed
-
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007;13(10):1219–1227. - PubMed
-
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev. Dyn. 1995;202(3):229–243. - PubMed
-
- Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers–Danlos-like changes in bone and other connective tissues. J. Bone Miner. Res. 2002;17(7):1180–1189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

