An early onset progressive motor neuron disorder in Scyl1-deficient mice is associated with mislocalization of TDP-43
- PMID: 23175812
- PMCID: PMC6621767
- DOI: 10.1523/JNEUROSCI.1787-12.2012
An early onset progressive motor neuron disorder in Scyl1-deficient mice is associated with mislocalization of TDP-43
Abstract
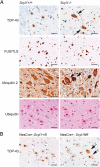
The molecular and cellular bases of motor neuron diseases (MNDs) are still poorly understood. The diseases are mostly sporadic, with ~10% of cases being familial. In most cases of familial motor neuronopathy, the disease is caused by either gain-of-adverse-effect mutations or partial loss-of-function mutations in ubiquitously expressed genes that serve essential cellular functions. Here we show that deletion of Scyl1, an evolutionarily conserved and ubiquitously expressed gene encoding the COPI-associated protein pseudokinase SCYL1, causes an early onset progressive MND with characteristic features of amyotrophic lateral sclerosis (ALS). Skeletal muscles of Scyl1(-/-) mice displayed neurogenic atrophy, fiber type switching, and disuse atrophy. Peripheral nerves showed axonal degeneration. Loss of lower motor neurons (LMNs) and large-caliber axons was conspicuous in Scyl1(-/-) animals. Signs of neuroinflammation were seen throughout the CNS, most notably in the ventral horn of the spinal cord. Neural-specific, but not skeletal muscle-specific, deletion of Scyl1 was sufficient to cause motor dysfunction, indicating that SCYL1 acts in a neural cell-autonomous manner to prevent LMN degeneration and motor functions. Remarkably, deletion of Scyl1 resulted in the mislocalization and accumulation of TDP-43 (TAR DNA-binding protein of 43 kDa) and ubiquilin 2 into cytoplasmic inclusions within LMNs, features characteristic of most familial and sporadic forms of ALS. Together, our results identify SCYL1 as a key regulator of motor neuron survival, and Scyl1(-/-) mice share pathological features with many human neurodegenerative conditions.
Figures

Similar articles
-
Overlapping Role of SCYL1 and SCYL3 in Maintaining Motor Neuron Viability.J Neurosci. 2018 Mar 7;38(10):2615-2630. doi: 10.1523/JNEUROSCI.2282-17.2018. Epub 2018 Feb 7. J Neurosci. 2018. PMID: 29437892 Free PMC article.
-
The Overexpression of TDP-43 Protein in the Neuron and Oligodendrocyte Cells Causes the Progressive Motor Neuron Degeneration in the SOD1 G93A Transgenic Mouse Model of Amyotrophic Lateral Sclerosis.Int J Biol Sci. 2016 Aug 15;12(9):1140-9. doi: 10.7150/ijbs.15938. eCollection 2016. Int J Biol Sci. 2016. PMID: 27570488 Free PMC article.
-
Lower motor neuron involvement in TAR DNA-binding protein of 43 kDa-related frontotemporal lobar degeneration and amyotrophic lateral sclerosis.JAMA Neurol. 2014 Feb;71(2):172-9. doi: 10.1001/jamaneurol.2013.5489. JAMA Neurol. 2014. PMID: 24378564
-
Autophagy in motor neuron diseases.Prog Mol Biol Transl Sci. 2020;172:157-202. doi: 10.1016/bs.pmbts.2020.03.009. Epub 2020 Apr 24. Prog Mol Biol Transl Sci. 2020. PMID: 32620242 Review.
-
[Clinical and pathological spectrum of TDP-43 associated ALS].Rinsho Shinkeigaku. 2010 Nov;50(11):940-2. doi: 10.5692/clinicalneurol.50.940. Rinsho Shinkeigaku. 2010. PMID: 21921519 Review. Japanese.
Cited by
-
SCYL2 Protects CA3 Pyramidal Neurons from Excitotoxicity during Functional Maturation of the Mouse Hippocampus.J Neurosci. 2015 Jul 22;35(29):10510-22. doi: 10.1523/JNEUROSCI.2056-14.2015. J Neurosci. 2015. PMID: 26203146 Free PMC article.
-
The intronic branch point sequence is under strong evolutionary constraint in the bovine and human genome.Commun Biol. 2021 Oct 21;4(1):1206. doi: 10.1038/s42003-021-02725-7. Commun Biol. 2021. PMID: 34675361 Free PMC article.
-
Dilysine motifs in exon 2b of SMN protein mediate binding to the COPI vesicle protein α-COP and neurite outgrowth in a cell culture model of spinal muscular atrophy.Hum Mol Genet. 2013 Oct 15;22(20):4043-52. doi: 10.1093/hmg/ddt254. Epub 2013 May 31. Hum Mol Genet. 2013. PMID: 23727837 Free PMC article.
-
Variant in SCYL1 gene causes aberrant splicing in a family with cerebellar ataxia, recurrent episodes of liver failure, and growth retardation.Eur J Hum Genet. 2019 Feb;27(2):263-268. doi: 10.1038/s41431-018-0268-2. Epub 2018 Sep 26. Eur J Hum Genet. 2019. PMID: 30258122 Free PMC article.
-
SCYL pseudokinases in neuronal function and survival.Neural Regen Res. 2016 Jan;11(1):42-4. doi: 10.4103/1673-5374.175040. Neural Regen Res. 2016. PMID: 26981075 Free PMC article. Review.
References
-
- Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119:2173–2183. - PubMed
-
- Blot S, Poirier C, Dreyfus PA. The mouse mutation muscle deficient (mdf) is characterized by a progressive motoneuron disease. J Neuropathol Exp Neurol. 1995;54:812–825. - PubMed
-
- Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–36343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
