Excessive bone formation in a mouse model of ankylosing spondylitis is associated with decreases in Wnt pathway inhibitors
- PMID: 23171658
- PMCID: PMC3674607
- DOI: 10.1186/ar4096
Excessive bone formation in a mouse model of ankylosing spondylitis is associated with decreases in Wnt pathway inhibitors
Abstract
Introduction: Ankylosing spondylitis (AS) is unique in its pathology where inflammation commences at the entheses before progressing to an osteoproliferative phenotype generating excessive bone formation that can result in joint fusion. The underlying mechanisms of this progression are poorly understood. Recent work has suggested that changes in Wnt signalling, a key bone regulatory pathway, may contribute to joint ankylosis in AS. Using the proteoglycan-induced spondylitis (PGISp) mouse model which displays spondylitis and eventual joint fusion following an initial inflammatory stimulus, we have characterised the structural and molecular changes that underlie disease progression.
Methods: PGISp mice were characterised 12 weeks after initiation of inflammation using histology, immunohistochemistry (IHC) and expression profiling.
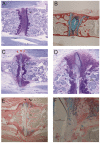
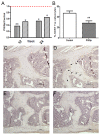
Results: Inflammation initiated at the periphery of the intervertebral discs progressing to disc destruction followed by massively excessive cartilage and bone matrix formation, as demonstrated by toluidine blue staining and IHC for collagen type I and osteocalcin, leading to syndesmophyte formation. Expression levels of DKK1 and SOST, Wnt signalling inhibitors highly expressed in joints, were reduced by 49% and 63% respectively in the spine PGISp compared with control mice (P < 0.05) with SOST inhibition confirmed by IHC. Microarray profiling showed genes involved in inflammation and immune-regulation were altered. Further, a number of genes specifically involved in bone regulation including other members of the Wnt pathway were also dysregulated.
Conclusions: This study implicates the Wnt pathway as a likely mediator of the mechanism by which inflammation induces bony ankylosis in spondyloarthritis, raising the potential that therapies targeting this pathway may be effective in preventing this process.
Figures



Similar articles
-
Inflammation-driven bone formation in a mouse model of ankylosing spondylitis: sequential not parallel processes.Arthritis Res Ther. 2016 Jan 29;18:35. doi: 10.1186/s13075-015-0805-0. Arthritis Res Ther. 2016. PMID: 26831337 Free PMC article.
-
Early anti-inflammatory intervention ameliorates axial disease in the proteoglycan-induced spondylitis mouse model of ankylosing spondylitis.BMC Musculoskelet Disord. 2017 May 30;18(1):228. doi: 10.1186/s12891-017-1600-7. BMC Musculoskelet Disord. 2017. PMID: 28558827 Free PMC article.
-
Inflammation Intensity-Dependent Expression of Osteoinductive Wnt Proteins Is Critical for Ectopic New Bone Formation in Ankylosing Spondylitis.Arthritis Rheumatol. 2018 Jul;70(7):1056-1070. doi: 10.1002/art.40468. Epub 2018 May 7. Arthritis Rheumatol. 2018. PMID: 29481736
-
Wnt signaling in ankylosing spondylitis.Clin Rheumatol. 2014 Jun;33(6):759-62. doi: 10.1007/s10067-014-2663-6. Epub 2014 May 13. Clin Rheumatol. 2014. PMID: 24820146 Review.
-
Ankylosis in ankylosing spondylitis: current concepts.Clin Rheumatol. 2015 Jun;34(6):1003-7. doi: 10.1007/s10067-015-2956-4. Clin Rheumatol. 2015. PMID: 25935456 Review.
Cited by
-
Immune cell transcript modules reveal leukocyte heterogeneity in synovial biopsies of seronegative spondylarthropathy patients.BMC Musculoskelet Disord. 2014 Dec 19;15:446. doi: 10.1186/1471-2474-15-446. BMC Musculoskelet Disord. 2014. PMID: 25526985 Free PMC article.
-
Identification of potential transcriptomic markers in developing ankylosing spondylitis: a meta-analysis of gene expression profiles.Biomed Res Int. 2015;2015:826316. doi: 10.1155/2015/826316. Epub 2015 Jan 22. Biomed Res Int. 2015. PMID: 25688367 Free PMC article.
-
Mediators of inflammation and bone remodeling in rheumatic disease.Semin Cell Dev Biol. 2016 Jan;49:2-10. doi: 10.1016/j.semcdb.2015.10.013. Epub 2015 Oct 19. Semin Cell Dev Biol. 2016. PMID: 26481971 Free PMC article. Review.
-
The osteogenic potential of ligament fibroblasts is greater in ankylosing spondylitis patients than in patients with osteoarthritis.Z Rheumatol. 2015 May;74(4):340-5. doi: 10.1007/s00393-014-1394-z. Z Rheumatol. 2015. PMID: 25876050 Clinical Trial.
-
Regulatory role of capsaicin-sensitive peptidergic sensory nerves in the proteoglycan-induced autoimmune arthritis model of the mouse.J Neuroinflammation. 2018 Dec 3;15(1):335. doi: 10.1186/s12974-018-1364-5. J Neuroinflammation. 2018. PMID: 30509328 Free PMC article.
References
-
- Ronneberger M, Schett G. Pathophysiology of Spondyloarthritis. Current Rheumatology Reports. 2011. pp. 1–5. - PubMed
-
- Appel H, Loddenkemper C, Miossec P. Rheumatoid arthritis and ankylosing spondylitis - pathology of acute inflammation. Clin Exp Rheumatol. 2009;14:S15–19. - PubMed
-
- Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Ostergaard M, Lambert RG. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009;14:93–102. doi: 10.1002/art.24132. - DOI - PubMed
-
- Pedersen SJ, Sørensen IJ, Lambert RGW, Hermann K-GA, Garnero P, Johansen JS, Madsen OR, Hansen A, Hansen MS, Thamsborg G, Andersen LS, Majgaard O, Loft AG, Erlendsson J, Asmussen KH, Jurik AG, Møller J, Hasselquist M, Mikkelsen D, Østergaard M. Radiographic progression is associated with resolution of systemic inflammation in patients with axial spondylarthritis treated with tumor necrosis factor α inhibitors: a study of radiographic progression, inflammation on magnetic resonance imaging, and circulating biomarkers of inflammation, angiogenesis, and cartilage and bone turnover. Arthritis Rheum. 2011;14:3789–3800. doi: 10.1002/art.30627. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

