Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model
- PMID: 23169917
- PMCID: PMC3501977
- DOI: 10.1093/brain/aws268
Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model
Abstract
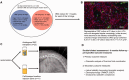
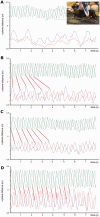
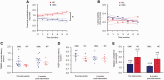
This study was designed to determine whether an intervention proven effective in the laboratory to ameliorate the effects of experimental spinal cord injury could provide sufficient benefit to be of value to clinical cases. Intraspinal olfactory ensheathing cell transplantation improves locomotor outcome after spinal cord injury in 'proof of principle' experiments in rodents, suggesting the possibility of efficacy in human patients. However, laboratory animal spinal cord injury cannot accurately model the inherent heterogeneity of clinical patient cohorts, nor are all aspects of their spinal cord function readily amenable to objective evaluation. Here, we measured the effects of intraspinal transplantation of cells derived from olfactory mucosal cultures (containing a mean of ~50% olfactory ensheathing cells) in a population of spinal cord-injured companion dogs that accurately model many of the potential obstacles involved in transition from laboratory to clinic. Dogs with severe chronic thoracolumbar spinal cord injuries (equivalent to ASIA grade 'A' human patients at ~12 months after injury) were entered into a randomized double-blinded clinical trial in which they were allocated to receive either intraspinal autologous cells derived from olfactory mucosal cultures or injection of cell transport medium alone. Recipients of olfactory mucosal cell transplants gained significantly better fore-hind coordination than those dogs receiving cell transport medium alone. There were no significant differences in outcome between treatment groups in measures of long tract functionality. We conclude that intraspinal olfactory mucosal cell transplantation improves communication across the damaged region of the injured spinal cord, even in chronically injured individuals. However, we find no evidence for concomitant improvement in long tract function.
Figures

Comment in
-
Towards treating spinal cord injury in 'patients': one step at a time.Brain. 2012 Nov;135(Pt 11):3203-5. doi: 10.1093/brain/aws294. Brain. 2012. PMID: 23169916 No abstract available.
Similar articles
-
Transplantation of encapsulated autologous olfactory ensheathing cell populations expressing chondroitinase for spinal cord injury: A safety and feasibility study in companion dogs.J Tissue Eng Regen Med. 2022 Sep;16(9):788-798. doi: 10.1002/term.3328. Epub 2022 Jun 10. J Tissue Eng Regen Med. 2022. PMID: 35686704 Free PMC article.
-
Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury.Cell Transplant. 2013;22(9):1591-612. doi: 10.3727/096368912X663532. Cell Transplant. 2013. PMID: 24007776 Clinical Trial.
-
Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial.Brain. 2008 Sep;131(Pt 9):2376-86. doi: 10.1093/brain/awn173. Epub 2008 Aug 8. Brain. 2008. PMID: 18689435 Free PMC article. Clinical Trial.
-
Olfactory ensheathing cells: their potential use for repairing the injured spinal cord.Spine (Phila Pa 1976). 2002 Apr 15;27(8):887-92. doi: 10.1097/00007632-200204150-00021. Spine (Phila Pa 1976). 2002. PMID: 11935115 Review.
-
Olfactory ensheathing cells and spinal cord repair.Keio J Med. 2005 Mar;54(1):8-14. doi: 10.2302/kjm.54.8. Keio J Med. 2005. PMID: 15832075 Review.
Cited by
-
A Novel Approach for Mucosal and Bulbar Olfactory Ensheathing Cells Isolation Based on the Non-adherent Subculture Technique.Basic Clin Neurosci. 2024 Mar-Apr;15(2):211-220. doi: 10.32598/bcn.2022.3579.1. Epub 2024 Mar 1. Basic Clin Neurosci. 2024. PMID: 39228451 Free PMC article.
-
Safety of Gonadal Tissue-Derived Mesenchymal Stem Cell Therapy in Geriatric Dogs with Chronic Disease.Animals (Basel). 2024 Jul 22;14(14):2134. doi: 10.3390/ani14142134. Animals (Basel). 2024. PMID: 39061596 Free PMC article.
-
Treatment of spinal cord injury with biomaterials and stem cell therapy in non-human primates and humans.Neural Regen Res. 2025 Feb 1;20(2):343-353. doi: 10.4103/NRR.NRR-D-23-01752. Epub 2024 May 24. Neural Regen Res. 2025. PMID: 38819038 Free PMC article.
-
Quantification of spinal ataxia in dogs with thoracolumbar spinal cord injury.Front Vet Sci. 2023 Aug 8;10:1183755. doi: 10.3389/fvets.2023.1183755. eCollection 2023. Front Vet Sci. 2023. PMID: 37614460 Free PMC article.
-
Transplantation of encapsulated autologous olfactory ensheathing cell populations expressing chondroitinase for spinal cord injury: A safety and feasibility study in companion dogs.J Tissue Eng Regen Med. 2022 Sep;16(9):788-798. doi: 10.1002/term.3328. Epub 2022 Jun 10. J Tissue Eng Regen Med. 2022. PMID: 35686704 Free PMC article.
References
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. - PubMed
-
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83. - PubMed
-
- Barnett SC, Riddell JS. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair—what can it achieve? Nat Clin Pract Neurol. 2007;3:152–61. - PubMed
-
- Begley CG, Ellis LM. Drug development: raise standards for preclinical cancer research. Nature. 2012;483:531–3. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

