Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β
- PMID: 23160153
- PMCID: PMC3721324
- DOI: 10.1038/ni.2474
Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β
Abstract
Interleukin 1 (IL-1) is an important mediator of innate immunity but can also promote inflammatory tissue damage. During chronic infections such as tuberculosis, the beneficial antimicrobial role of IL-1 must be balanced with the need to prevent immunopathology. By exogenously controlling the replication of Mycobacterium tuberculosis in vivo, we obviated the requirement for antimicrobial immunity and discovered that both IL-1 production and infection-induced immunopathology were suppressed by lymphocyte-derived interferon-γ (IFN-γ). This effect was mediated by nitric oxide (NO), which we found specifically inhibited assembly of the NLRP3 inflammasome via thiol nitrosylation. Our data indicate that the NO produced as a result of adaptive immunity is indispensable in modulating the destructive innate inflammatory responses elicited during persistent infections.
Figures

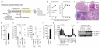
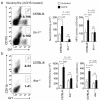

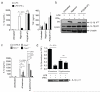
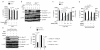

Comment in
-
Inflammasome: Turning on and off NLRP3.Nat Rev Immunol. 2013 Jan;13(1):1. doi: 10.1038/nri3366. Epub 2012 Nov 30. Nat Rev Immunol. 2013. PMID: 23197112 No abstract available.
-
Just say NO to NLRP3.Nat Immunol. 2013 Jan;14(1):12-4. doi: 10.1038/ni.2493. Nat Immunol. 2013. PMID: 23238751 No abstract available.
Similar articles
-
Just say NO to NLRP3.Nat Immunol. 2013 Jan;14(1):12-4. doi: 10.1038/ni.2493. Nat Immunol. 2013. PMID: 23238751 No abstract available.
-
Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-κB innate response pathways.J Virol. 2011 Dec;85(23):12505-17. doi: 10.1128/JVI.00410-11. Epub 2011 Sep 28. J Virol. 2011. PMID: 21957307 Free PMC article.
-
Brucella abortus nitric oxide metabolite regulates inflammasome activation and IL-1β secretion in murine macrophages.Eur J Immunol. 2019 Jul;49(7):1023-1037. doi: 10.1002/eji.201848016. Epub 2019 Apr 3. Eur J Immunol. 2019. PMID: 30919410 Free PMC article.
-
Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis.Eur J Immunol. 2012 Feb;42(2):374-84. doi: 10.1002/eji.201141548. Epub 2011 Dec 20. Eur J Immunol. 2012. PMID: 22101787
-
Biology of IL-27 and its role in the host immunity against Mycobacterium tuberculosis.Int J Biol Sci. 2015 Jan 5;11(2):168-75. doi: 10.7150/ijbs.10464. eCollection 2015. Int J Biol Sci. 2015. PMID: 25561899 Free PMC article. Review.
Cited by
-
Immune regulation and emerging roles of noncoding RNAs in Mycobacterium tuberculosis infection.Front Immunol. 2022 Oct 13;13:987018. doi: 10.3389/fimmu.2022.987018. eCollection 2022. Front Immunol. 2022. PMID: 36311754 Free PMC article. Review.
-
The intracellular environment of human macrophages that produce nitric oxide promotes growth of mycobacteria.Infect Immun. 2013 Sep;81(9):3198-209. doi: 10.1128/IAI.00611-13. Epub 2013 Jun 17. Infect Immun. 2013. PMID: 23774601 Free PMC article.
-
Natural Compounds as Regulators of NLRP3 Inflammasome-Mediated IL-1β Production.Mediators Inflamm. 2016;2016:5460302. doi: 10.1155/2016/5460302. Epub 2016 Sep 8. Mediators Inflamm. 2016. PMID: 27672241 Free PMC article. Review.
-
Persistent effects of Libby amphibole and amosite asbestos following subchronic inhalation in rats.Part Fibre Toxicol. 2016 Apr 15;13:17. doi: 10.1186/s12989-016-0130-z. Part Fibre Toxicol. 2016. PMID: 27083413 Free PMC article.
-
The role of the inflammasome in cardiovascular diseases.J Mol Med (Berl). 2014 Apr;92(4):307-19. doi: 10.1007/s00109-014-1144-3. Epub 2014 Mar 19. J Mol Med (Berl). 2014. PMID: 24638861 Review.
References
-
- Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. - PubMed
-
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. - PubMed
-
- Dinarello CA. Role of interleukin-1 in infectious diseases. Immunol Rev. 1992;127:119–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

