Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis
- PMID: 23159881
- PMCID: PMC3589822
- DOI: 10.1038/nbt.2434
Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis
Abstract
Aberrant T-cell activation underlies many autoimmune disorders, yet most attempts to induce T-cell tolerance have failed. Building on previous strategies for tolerance induction that exploited natural mechanisms for clearing apoptotic debris, we show that antigen-decorated microparticles (500-nm diameter) induce long-term T-cell tolerance in mice with relapsing experimental autoimmune encephalomyelitis. Specifically, intravenous infusion of either polystyrene or biodegradable poly(lactide-co-glycolide) microparticles bearing encephalitogenic peptides prevents the onset and modifies the course of the disease. These beneficial effects require microparticle uptake by marginal zone macrophages expressing the scavenger receptor MARCO and are mediated in part by the activity of regulatory T cells, abortive T-cell activation and T-cell anergy. Together these data highlight the potential for using microparticles to target natural apoptotic clearance pathways to inactivate pathogenic T cells and halt the disease process in autoimmunity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

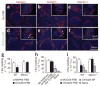

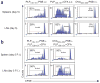
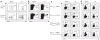

Comment in
-
Immunomodulators: particulate promotion of tolerance.Nat Rev Drug Discov. 2013 Jan;12(1):22-3. doi: 10.1038/nrd3916. Epub 2012 Dec 14. Nat Rev Drug Discov. 2013. PMID: 23237919 No abstract available.
-
Autoimmunity: Particulate promotion of tolerance.Nat Rev Immunol. 2013 Jan;13(1):6-7. doi: 10.1038/nri3373. Epub 2012 Dec 14. Nat Rev Immunol. 2013. PMID: 23237965 No abstract available.
Similar articles
-
Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis.J Immunol. 2000 Jan 15;164(2):670-8. doi: 10.4049/jimmunol.164.2.670. J Immunol. 2000. PMID: 10623809
-
Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells.J Immunol. 2011 Sep 1;187(5):2405-17. doi: 10.4049/jimmunol.1004175. Epub 2011 Aug 5. J Immunol. 2011. PMID: 21821796 Free PMC article.
-
The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance.Eur J Immunol. 2002 Apr;32(4):972-81. doi: 10.1002/1521-4141(200204)32:4<972::AID-IMMU972>3.0.CO;2-M. Eur J Immunol. 2002. PMID: 11920563
-
T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire.Annu Rev Immunol. 2002;20:101-23. doi: 10.1146/annurev.immunol.20.081701.141316. Epub 2001 Oct 4. Annu Rev Immunol. 2002. PMID: 11861599 Review.
-
Exploiting apoptosis for therapeutic tolerance induction.J Immunol. 2013 Dec 1;191(11):5341-6. doi: 10.4049/jimmunol.1302070. J Immunol. 2013. PMID: 24244028 Free PMC article. Review.
Cited by
-
PLGA Particles in Immunotherapy.Pharmaceutics. 2023 Feb 11;15(2):615. doi: 10.3390/pharmaceutics15020615. Pharmaceutics. 2023. PMID: 36839937 Free PMC article. Review.
-
Treatment of experimental autoimmune encephalomyelitis by codelivery of disease associated Peptide and dexamethasone in acetalated dextran microparticles.Mol Pharm. 2014 Mar 3;11(3):828-35. doi: 10.1021/mp4005172. Epub 2014 Feb 4. Mol Pharm. 2014. PMID: 24433027 Free PMC article.
-
Peptide Immunotherapy for Type 1 Diabetes-Clinical Advances.Front Immunol. 2018 Feb 28;9:392. doi: 10.3389/fimmu.2018.00392. eCollection 2018. Front Immunol. 2018. PMID: 29541078 Free PMC article. Review.
-
Bioengineering strategies to accelerate stem cell therapeutics.Nature. 2018 May;557(7705):335-342. doi: 10.1038/s41586-018-0089-z. Epub 2018 May 16. Nature. 2018. PMID: 29769665 Free PMC article. Review.
-
Potential for Antigen-Specific Tolerizing Immunotherapy in Systematic Lupus Erythematosus.Front Immunol. 2021 Jul 16;12:654701. doi: 10.3389/fimmu.2021.654701. eCollection 2021. Front Immunol. 2021. PMID: 34335564 Free PMC article. Review.
References
-
- Christen U, von Herrath MG. Initiation of autoimmunity. Curr Opin Immunol. 2004;16:759–767. - PubMed
-
- Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. - PubMed
-
- Kohm AP, Turley DM, Miller SD. Targeting the TCR: T-cell receptor and peptide-specific tolerance-based strategies for restoring self-tolerance in CNS autoimmune disease. Int Rev Immunol. 2005;24:361–392. - PubMed
-
- Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

