Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly
- PMID: 23159225
- PMCID: PMC3538030
- DOI: 10.1016/j.immuni.2012.10.014
Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly
Abstract
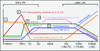
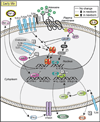
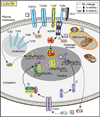
Given the "inborn" nature of the innate immune system, it is surprising to find that innate immune function does in fact change with age. Similar patterns of distinct Toll-like-receptor-mediated immune responses come to light when one contrasts innate immune development at the beginning of life with that toward the end of life. Importantly, these developmental patterns of innate cytokine responses correlate with clinical patterns of susceptibility to disease: A heightened risk of suffering from excessive inflammation is often detected in prematurely born infants, disappears over the first few months of life, and reappears toward the end of life. In addition, risk periods for particular infections in early life reemerge in older adults. The near-mirror-image patterns that emerge in contrasts of early versus late innate immune ontogeny emphasize changes in host-environment interactions as the underlying molecular and teleologic drivers.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells.PLoS One. 2010 Nov 30;5(11):e15041. doi: 10.1371/journal.pone.0015041. PLoS One. 2010. PMID: 21152080 Free PMC article.
-
Breastfeeding modulates neonatal innate immune responses: a prospective birth cohort study.Pediatr Allergy Immunol. 2012 Feb;23(1):65-74. doi: 10.1111/j.1399-3038.2011.01230.x. Epub 2011 Nov 22. Pediatr Allergy Immunol. 2012. PMID: 22103307
-
Environment impacts innate immune ontogeny.Innate Immun. 2017 Jan;23(1):3-10. doi: 10.1177/1753425916671018. Epub 2016 Oct 3. Innate Immun. 2017. PMID: 27697850
-
Innate immunity: toll-like receptors and some more. A brief history, basic organization and relevance for the human newborn.Neonatology. 2007;92(3):145-57. doi: 10.1159/000102054. Epub 2007 Apr 27. Neonatology. 2007. PMID: 17476116 Review.
-
Recent insights into the role of Toll-like receptors in viral infection.Clin Exp Immunol. 2010 Sep;161(3):397-406. doi: 10.1111/j.1365-2249.2010.04196.x. Clin Exp Immunol. 2010. PMID: 20560984 Free PMC article. Review.
Cited by
-
Maturation of the Acute Hepatic TLR4/NF-κB Mediated Innate Immune Response Is p65 Dependent in Mice.Front Immunol. 2020 Aug 21;11:1892. doi: 10.3389/fimmu.2020.01892. eCollection 2020. Front Immunol. 2020. PMID: 32973783 Free PMC article.
-
Decreased pattern recognition receptor signaling, interferon-signature, and bactericidal/permeability-increasing protein gene expression in cord blood of term low birth weight human newborns.PLoS One. 2013 Apr 23;8(4):e62845. doi: 10.1371/journal.pone.0062845. Print 2013. PLoS One. 2013. PMID: 23626859 Free PMC article.
-
Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking.PLoS Pathog. 2015 Jun 24;11(6):e1005004. doi: 10.1371/journal.ppat.1005004. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26107875 Free PMC article.
-
Transplacental Transmission of SARS-CoV-2: A Narrative Review.Medicina (Kaunas). 2024 Sep 18;60(9):1517. doi: 10.3390/medicina60091517. Medicina (Kaunas). 2024. PMID: 39336558 Free PMC article. Review.
-
Neonatal sepsis and cardiovascular dysfunction I: mechanisms and pathophysiology.Pediatr Res. 2024 Apr;95(5):1207-1216. doi: 10.1038/s41390-023-02926-2. Epub 2023 Dec 4. Pediatr Res. 2024. PMID: 38044334 Review.
References
-
- Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 2007a;178:6912–6922. - PubMed
-
- Aksoy E, Albarani V, Nguyen M, Laes JF, Ruelle JL, De Wit D, Willems F, Goldman M, Goriely S. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood. 2007;109:2887–2893. - PubMed
-
- Azizia M, Lloyd J, Allen M, Klein N, Peebles D. Immune status in very preterm neonates. Pediatrics. 2012;129:e967–e974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

