Crosstalk between ROR1 and the Pre-B cell receptor promotes survival of t(1;19) acute lymphoblastic leukemia
- PMID: 23153538
- PMCID: PMC3500515
- DOI: 10.1016/j.ccr.2012.08.027
Crosstalk between ROR1 and the Pre-B cell receptor promotes survival of t(1;19) acute lymphoblastic leukemia
Abstract
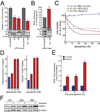
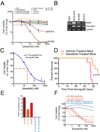
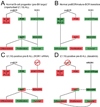
We report that t(1;19) ALL cells universally exhibit expression of and dependence on the cell surface receptor ROR1. We further identify t(1;19) ALL cell sensitivity to the kinase inhibitor dasatinib due to its inhibition of the pre-B cell receptor (pre-BCR) signaling complex. These phenotypes are a consequence of developmental arrest at an intermediate/late stage of B-lineage maturation. Additionally, inhibition of pre-BCR signaling induces further ROR1 upregulation, and we identify distinct ROR1 and pre-BCR downstream signaling pathways that are modulated in a counterbalancing manner-both leading to AKT phosphorylation. Consistent with this, AKT phosphorylation is transiently eliminated after dasatinib treatment, but is partially restored following dasatinib potentiation of ROR1 expression. Consequently, ROR1 silencing accentuates dasatinib killing of t(1;19) ALL cells.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Development of resistance to dasatinib in Bcr/Abl-positive acute lymphoblastic leukemia.Leukemia. 2010 Apr;24(4):813-20. doi: 10.1038/leu.2009.302. Epub 2010 Jan 28. Leukemia. 2010. PMID: 20111071 Free PMC article.
-
Wnt5a and ROR1 activate non-canonical Wnt signaling via RhoA in TCF3-PBX1 acute lymphoblastic leukemia and highlight new treatment strategies via Bcl-2 co-targeting.Oncogene. 2019 Apr;38(17):3288-3300. doi: 10.1038/s41388-018-0670-9. Epub 2019 Jan 10. Oncogene. 2019. PMID: 30631148
-
ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia.Leuk Res. 2011 Oct;35(10):1390-4. doi: 10.1016/j.leukres.2011.06.021. Epub 2011 Aug 2. Leuk Res. 2011. PMID: 21813176 Free PMC article.
-
Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.Clin Ther. 2007 Nov;29(11):2289-308. doi: 10.1016/j.clinthera.2007.11.005. Clin Ther. 2007. PMID: 18158072 Review.
-
Targeting ROR1 identifies new treatment strategies in hematological cancers.Biochem Soc Trans. 2017 Apr 15;45(2):457-464. doi: 10.1042/BST20160272. Biochem Soc Trans. 2017. PMID: 28408486 Review.
Cited by
-
Stable silencing of ROR1 regulates cell cycle, apoptosis, and autophagy in a lung adenocarcinoma cell line.Int J Clin Exp Pathol. 2020 May 1;13(5):1108-1120. eCollection 2020. Int J Clin Exp Pathol. 2020. PMID: 32509086 Free PMC article.
-
ROR1, an embryonic protein with an emerging role in cancer biology.Protein Cell. 2014 Jul;5(7):496-502. doi: 10.1007/s13238-014-0059-7. Epub 2014 Apr 22. Protein Cell. 2014. PMID: 24752542 Free PMC article. Review.
-
Pre-B acute lymphoblastic leukemia with t(1;19) in an adult initially presenting as hematuria and bilateral renal enlargement: a case report and literature review.Clin Nephrol Case Stud. 2017 Sep 15;5:60-65. doi: 10.5414/CNCS109113. eCollection 2017. Clin Nephrol Case Stud. 2017. PMID: 29043149 Free PMC article.
-
Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review).Int J Oncol. 2017 Nov;51(5):1357-1369. doi: 10.3892/ijo.2017.4129. Epub 2017 Sep 19. Int J Oncol. 2017. PMID: 29048660 Free PMC article. Review.
-
A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis.Nat Cell Biol. 2017 Feb;19(2):106-119. doi: 10.1038/ncb3464. Epub 2017 Jan 23. Nat Cell Biol. 2017. PMID: 28114269 Free PMC article.
References
-
- Baskar S, Kwong KY, Hofer T, Levy JM, Kennedy MG, Lee E, Staudt LM, Wilson WH, Wiestner A, Rader C. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2008;14:396–404. - PubMed
-
- Bhuin T, Roy JK. Rab11 regulates JNK and Raf/MAPK-ERK signalling pathways during Drosophila wing development. Cell Biol Int. 2010;34:1113–1118. - PubMed
-
- Bilodeau MT, Balitza AE, Hoffman JM, Manley PJ, Barnett SF, Defeo-Jones D, Haskell K, Jones RE, Leander K, Robinson RG, et al. Allosteric inhibitors of Akt1 and Akt2: a naphthyridinone with efficacy in an A2780 tumor xenograft model. Bioorg Med Chem Lett. 2008;18:3178–3182. - PubMed
-
- Boulos N, Mulder HL, Calabrese CR, Morrison JB, Rehg JE, Relling MV, Sherr CJ, Williams RT. Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR-ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;117:3585–3595. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

