Semi-artificial mouse skin membrane feeding technique for adult tick, Haemaphysalis longicornis
- PMID: 23153119
- PMCID: PMC3514109
- DOI: 10.1186/1756-3305-5-263
Semi-artificial mouse skin membrane feeding technique for adult tick, Haemaphysalis longicornis
Abstract
Background: An in vitro artificial feeding technique for hard ticks is quite useful for studying the tick-pathogen interactions. Here, we report a novel semi-artificial feeding technique for the adult parthenogenetic tick, Haemaphysalis longicornis, using mouse skin membrane.
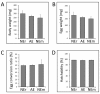
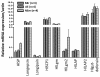
Findings: Skin with attached adult ticks was removed from the mouse body at 4 to 5 days post-infestation for the construction of the feeding system. This system supplied with rabbit blood was kept in >95% relative humidity at 30°C during the feeding, and ticks were fully engorged (artificially engorged, AE) within 12 to 48 h. For comparison, ticks were fed to engorgement solely on rabbit or mouse for 5 days as controls (naturally engorged on rabbit, NEr, or mouse, NEm). Blood digestion-related gene expression in the midgut and reproductive fitness were compared. Body weight, egg mass weight, egg conversion ratio, and hatchability of eggs did not show any significant differences. We analyzed transcription profiles of selected genes assayed by quantitative RT-PCR and revealed similar patterns of expression between NEr and AE but some differences between NEm and AE or NEm and NEr.
Conclusions: Our results demonstrate that this semi-artificial feeding technique mimics natural feeding processes of ticks and can be utilized as a standardized method to inoculate pathogens, especially Babesia protozoa, into H. longicornis and possibly other tick species as well.
Figures


Similar articles
-
Transovarial persistence of Babesia ovata DNA in a hard tick, Haemaphysalis longicornis, in a semi-artificial mouse skin membrane feeding system.Acta Parasitol. 2017 Dec 20;62(4):836-841. doi: 10.1515/ap-2017-0100. Acta Parasitol. 2017. PMID: 29035855
-
Localized expression and inhibition effect of miR-184 on blood digestion and oviposition in Haemaphysalis longicornis (Acari: Ixodidae).Parasit Vectors. 2019 Oct 25;12(1):500. doi: 10.1186/s13071-019-3754-7. Parasit Vectors. 2019. PMID: 31653232 Free PMC article.
-
Haemaphysalis longicornis calreticulin is not an effective molecular tool for tick bite diagnosis and disruption of tick infestations.Vet Parasitol. 2022 Sep;309:109775. doi: 10.1016/j.vetpar.2022.109775. Epub 2022 Aug 4. Vet Parasitol. 2022. PMID: 35939902
-
Ultrastructure features of the midgut of the female adult Amblyomma cajennense ticks Fabricius, 1787 (Acari: Ixodidae) in several feeding stages and subjected to three infestations.Micron. 2010 Oct;41(7):710-21. doi: 10.1016/j.micron.2010.05.015. Epub 2010 Jun 4. Micron. 2010. PMID: 20580564 Review.
-
In vitro feeding assays for hard ticks.Trends Parasitol. 2007 Sep;23(9):445-9. doi: 10.1016/j.pt.2007.07.010. Epub 2007 Jul 27. Trends Parasitol. 2007. PMID: 17681859 Review.
Cited by
-
The widely distributed hard tick, Haemaphysalis longicornis, can retain canine parvovirus, but not be infected in laboratory condition.J Vet Med Sci. 2015 Apr;77(4):405-11. doi: 10.1292/jvms.14-0199. Epub 2014 Dec 19. J Vet Med Sci. 2015. PMID: 25650060 Free PMC article.
-
Ambivalent Roles of Oxidative Stress in Triangular Relationships among Arthropod Vectors, Pathogens and Hosts.Antioxidants (Basel). 2022 Jun 25;11(7):1254. doi: 10.3390/antiox11071254. Antioxidants (Basel). 2022. PMID: 35883744 Free PMC article. Review.
-
RNA activation in ticks.Sci Rep. 2023 Jun 8;13(1):9341. doi: 10.1038/s41598-023-36523-4. Sci Rep. 2023. PMID: 37291173 Free PMC article.
-
Tick-Host Range Adaptation: Changes in Protein Profiles in Unfed Adult Ixodes scapularis and Amblyomma americanum Saliva Stimulated to Feed on Different Hosts.Front Cell Infect Microbiol. 2017 Dec 19;7:517. doi: 10.3389/fcimb.2017.00517. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29312895 Free PMC article.
-
Artificial Feeding Systems for Vector-Borne Disease Studies.Biology (Basel). 2024 Mar 15;13(3):188. doi: 10.3390/biology13030188. Biology (Basel). 2024. PMID: 38534457 Free PMC article. Review.
References
-
- Kröber T, Guerin P. The tick blood meal: from a living animal or from a silicone membrane? ALTEX. 2007;Spec Issue:39–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

