AlkB dioxygenase preferentially repairs protonated substrates: specificity against exocyclic adducts and molecular mechanism of action
- PMID: 23148216
- PMCID: PMC3537041
- DOI: 10.1074/jbc.M112.353342
AlkB dioxygenase preferentially repairs protonated substrates: specificity against exocyclic adducts and molecular mechanism of action
Abstract
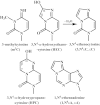
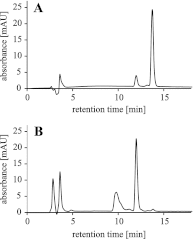
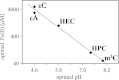
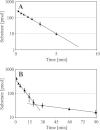
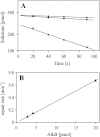
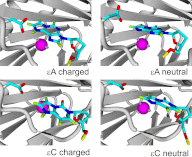
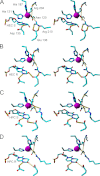
Efficient repair by Escherichia coli AlkB dioxygenase of exocyclic DNA adducts 3,N(4)-ethenocytosine, 1,N(6)-ethenoadenine, 3,N(4)-α-hydroxyethanocytosine, and reported here for the first time 3,N(4)-α-hydroxypropanocytosine requires higher Fe(II) concentration than the reference 3-methylcytosine. The pH optimum for the repair follows the order of pK(a) values for protonation of the adduct, suggesting that positively charged substrates favorably interact with the negatively charged carboxylic group of Asp-135 side chain in the enzyme active center. This interaction is supported by molecular modeling, indicating that 1,N(6)-ethenoadenine and 3,N(4)-ethenocytosine are bound to AlkB more favorably in their protonated cationic forms. An analysis of the pattern of intermolecular interactions that stabilize the location of the ligand points to a role of Asp-135 in recognition of the adduct in its protonated form. Moreover, ab initio calculations also underline the role of substrate protonation in lowering the free energy barrier of the transition state of epoxidation of the etheno adducts studied. The observed time courses of repair of mixtures of stereoisomers of 3,N(4)-α-hydroxyethanocytosine or 3,N(4)-α-hydroxypropanocytosine are unequivocally two-exponential curves, indicating that the respective isomers are repaired by AlkB with different efficiencies. Molecular modeling of these adducts bound by AlkB allowed evaluation of the participation of their possible conformational states in the enzymatic reaction.
Figures

Similar articles
-
Insights into the Direct Oxidative Repair of Etheno Lesions: MD and QM/MM Study on the Substrate Scope of ALKBH2 and AlkB.DNA Repair (Amst). 2020 Dec;96:102944. doi: 10.1016/j.dnarep.2020.102944. Epub 2020 Sep 9. DNA Repair (Amst). 2020. PMID: 33161373 Free PMC article.
-
Chloroacetaldehyde-induced mutagenesis in Escherichia coli: the role of AlkB protein in repair of 3,N(4)-ethenocytosine and 3,N(4)-alpha-hydroxyethanocytosine.Mutat Res. 2010 Feb 3;684(1-2):24-34. doi: 10.1016/j.mrfmmm.2009.11.005. Epub 2009 Nov 24. Mutat Res. 2010. PMID: 19941873
-
1,N6-α-hydroxypropanoadenine, the acrolein adduct to adenine, is a substrate for AlkB dioxygenase.Biochem J. 2017 May 16;474(11):1837-1852. doi: 10.1042/BCJ20161008. Biochem J. 2017. PMID: 28408432
-
The AlkB Family of Fe(II)/α-Ketoglutarate-dependent Dioxygenases: Repairing Nucleic Acid Alkylation Damage and Beyond.J Biol Chem. 2015 Aug 21;290(34):20734-20742. doi: 10.1074/jbc.R115.656462. Epub 2015 Jul 7. J Biol Chem. 2015. PMID: 26152727 Free PMC article. Review.
-
Oxidative dealkylation DNA repair mediated by the mononuclear non-heme iron AlkB proteins.J Inorg Biochem. 2006 Apr;100(4):670-8. doi: 10.1016/j.jinorgbio.2005.12.018. Epub 2006 Feb 15. J Inorg Biochem. 2006. PMID: 16469386 Free PMC article. Review.
Cited by
-
Quantum mechanics/molecular mechanics study on the oxygen binding and substrate hydroxylation step in AlkB repair enzymes.Chemistry. 2014 Jan 7;20(2):435-46. doi: 10.1002/chem.201303282. Epub 2013 Dec 11. Chemistry. 2014. PMID: 24339041 Free PMC article.
-
Adaptive Response Enzyme AlkB Preferentially Repairs 1-Methylguanine and 3-Methylthymine Adducts in Double-Stranded DNA.Chem Res Toxicol. 2016 Apr 18;29(4):687-93. doi: 10.1021/acs.chemrestox.5b00522. Epub 2016 Mar 15. Chem Res Toxicol. 2016. PMID: 26919079 Free PMC article.
-
Structural basis for substrate discrimination by E. coli repair enzyme, AlkB.RSC Adv. 2018 Jan 3;8(3):1281-1291. doi: 10.1039/c7ra11333a. eCollection 2018 Jan 2. RSC Adv. 2018. PMID: 35540905 Free PMC article.
-
DNA Demethylation in the Processes of Repair and Epigenetic Regulation Performed by 2-Ketoglutarate-Dependent DNA Dioxygenases.Int J Mol Sci. 2021 Sep 29;22(19):10540. doi: 10.3390/ijms221910540. Int J Mol Sci. 2021. PMID: 34638881 Free PMC article. Review.
-
Kinetic Studies on the 2-Oxoglutarate/Fe(II)-Dependent Nucleic Acid Modifying Enzymes from the AlkB and TET Families.DNA (Basel). 2023 Jun;3(2):65-84. doi: 10.3390/dna3020005. Epub 2023 Mar 30. DNA (Basel). 2023. PMID: 38698914 Free PMC article.
References
-
- Bartsch H. (1999) Keynote address: exocyclic adducts as new risk markers for DNA damage in man. IARC Sci. Publ. 1–16 - PubMed
-
- Hang B. (2004) Repair of exocyclic DNA adducts: rings of complexity. BioEssays 26, 1195–1208 - PubMed
-
- Leonard N. J. (1984) Etheno-substituted nucleotides and coenzymes: fluorescence and biological activity. CRC Crit. Rev. Biochem. 15, 125–199 - PubMed
-
- Krzyzosiak W. J., Biernat J., Ciesiolka J., Gornicki P., Wiewiorowski M. (1979) Further studies on adenosine and cytidine reaction with chloroacetaldehyde, a new support for the carbinolamine structure of the stable reaction intermediate and its relevance to the reaction mechanism and tRNA modification. Pol. J. Chem. 53, 243–252
-
- Kuśmierek J. T., Singer B. (1982) Chloroacetaldehyde-treated ribo- and deoxyribopolynucleotides. 1. Reaction products. Biochemistry 21, 5717–5722 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

