The stability of complement-mediated bactericidal activity in human serum against Salmonella
- PMID: 23145102
- PMCID: PMC3493494
- DOI: 10.1371/journal.pone.0049147
The stability of complement-mediated bactericidal activity in human serum against Salmonella
Abstract
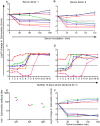
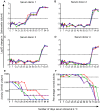
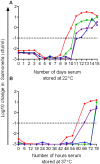
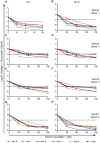
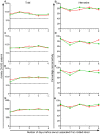
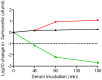
The complement cascade includes heat-labile proteins and care is required when handling serum in order to preserve its functional integrity. We have previously used a whole human serum bactericidal assay to show that antibody and an intact complement system are required in blood for killing of invasive isolates of Salmonella. The aim of the present study was to evaluate the conditions under which human serum can be stored and manipulated while maintaining complement integrity. Serum bactericidal activity against Salmonella was maintained for a minimum of 35 days when stored at 4°C, eight days at 22°C and 54 hours at 37°C. Up to three freeze-thaw cycles had no effect on the persistence of bactericidal activity and hemolytic complement assays confirmed no effect on complement function. Delay in the separation of serum for up to four days from clotted blood stored at 22°C did not affect bactericidal activity. Dilution of serum resulted in an increased rate of loss of bactericidal activity and so serum should be stored undiluted. These findings indicate that the current guidelines concerning manipulation and storage of human serum to preserve complement integrity and function leave a large margin for safety with regards to bactericidal activity against Salmonella. The study provides a scheme for determining the requirements for serum handling in relation to functional activity of complement in other systems.
Conflict of interest statement
Figures
Similar articles
-
Invasive African nontyphoidal Salmonella requires high levels of complement for cell-free antibody-dependent killing.J Immunol Methods. 2013 Jan 31;387(1-2):121-9. doi: 10.1016/j.jim.2012.10.005. Epub 2012 Oct 22. J Immunol Methods. 2013. PMID: 23085530
-
Absent bactericidal activity of mouse serum against invasive African nontyphoidal Salmonella results from impaired complement function but not a lack of antibody.J Immunol. 2011 Feb 15;186(4):2365-71. doi: 10.4049/jimmunol.1000284. Epub 2011 Jan 7. J Immunol. 2011. PMID: 21217014
-
Role of antilipopolysaccharide antibodies in serum bactericidal activity against Salmonella enterica serovar Typhimurium in healthy adults and children in the United States.Clin Vaccine Immunol. 2013 Oct;20(10):1491-8. doi: 10.1128/CVI.00289-13. Epub 2013 Jun 26. Clin Vaccine Immunol. 2013. PMID: 23803904 Free PMC article.
-
Serum bactericidal antibody assays - The role of complement in infection and immunity.Vaccine. 2015 Aug 26;33(36):4414-21. doi: 10.1016/j.vaccine.2015.07.019. Epub 2015 Jul 15. Vaccine. 2015. PMID: 26187262 Review.
-
[The efficiency of the bactericidal action of serum raised by complement and lysozyme against bacteria which avoid the immunological response of higher organisms].Postepy Hig Med Dosw (Online). 2009 Oct 19;63:471-84. Postepy Hig Med Dosw (Online). 2009. PMID: 19850971 Review. Polish.
Cited by
-
Direct enhancement of viral neutralising antibody potency by the complement system: a largely forgotten phenomenon.Cell Mol Life Sci. 2024 Jan 11;81(1):22. doi: 10.1007/s00018-023-05074-2. Cell Mol Life Sci. 2024. PMID: 38200235 Free PMC article. Review.
-
Putative Role of an ABC Efflux System in Aliarcobacter butzleri Resistance and Virulence.Antibiotics (Basel). 2023 Feb 6;12(2):339. doi: 10.3390/antibiotics12020339. Antibiotics (Basel). 2023. PMID: 36830250 Free PMC article.
-
Ecoimmunology in the field: Measuring multiple dimensions of immune function with minimally invasive, field-adapted techniques.Am J Hum Biol. 2022 Nov;34(11):e23784. doi: 10.1002/ajhb.23784. Epub 2022 Jul 21. Am J Hum Biol. 2022. PMID: 35861267 Free PMC article. Review.
-
A Comparative Study of Body Lice and Bed Bugs Reveals Factors Potentially Involved in Differential Vector Competence for the Relapsing Fever Spirochete Borrelia recurrentis.Infect Immun. 2022 May 19;90(5):e0068321. doi: 10.1128/iai.00683-21. Epub 2022 Apr 6. Infect Immun. 2022. PMID: 35384689 Free PMC article.
-
Snap-freezing in the Field: Effect of Sample Holding Time on Performance of Bactericidal Assays.Integr Comp Biol. 2022 Dec 30;62(6):1693-1699. doi: 10.1093/icb/icac007. Integr Comp Biol. 2022. PMID: 35294024 Free PMC article.
References
-
- Nuttall GHF (1888) Experimente uber die bacterienfeindlichen Einflusse des thierischen Korpers. Zeitschr f Hygiene iv: 353–394.
-
- Bordet J (1895) Les leucocytes et les propriétés actives du sérum chez les vaccinés. Ann Inst Pasteur 9: 462.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

