Usp14 deficiency increases tau phosphorylation without altering tau degradation or causing tau-dependent deficits
- PMID: 23144711
- PMCID: PMC3483306
- DOI: 10.1371/journal.pone.0047884
Usp14 deficiency increases tau phosphorylation without altering tau degradation or causing tau-dependent deficits
Abstract
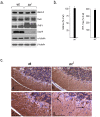
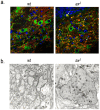
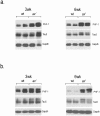
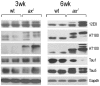
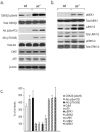
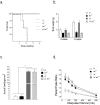
Regulated protein degradation by the proteasome plays an essential role in the enhancement and suppression of signaling pathways in the nervous system. Proteasome-associated factors are pivotal in ensuring appropriate protein degradation, and we have previously demonstrated that alterations in one of these factors, the proteasomal deubiquitinating enzyme ubiquitin-specific protease 14 (Usp14), can lead to proteasome dysfunction and neurological disease. Recent studies in cell culture have shown that Usp14 can also stabilize the expression of over-expressed, disease-associated proteins such as tau and ataxin-3. Using Usp14-deficient ax(J) mice, we investigated if loss of Usp14 results in decreased levels of endogenous tau and ataxin-3 in the nervous system of mice. Although loss of Usp14 did not alter the overall neuronal levels of tau and ataxin-3, we found increased levels of phosphorylated tau that correlated with the onset of axonal varicosities in the Usp14-deficient mice. These changes in tau phosphorylation were accompanied by increased levels of activated phospho-Akt, phosphorylated MAPKs, and inactivated phospho-GSK3β. However, genetic ablation of tau did not alter any of the neurological deficits in the Usp14-deficient mice, demonstrating that increased levels of phosphorylated tau do not necessarily lead to neurological disease. Due to the widespread activation of intracellular signaling pathways induced by the loss of Usp14, a better understanding of the cellular pathways regulated by the proteasome is required before effective proteasomal-based therapies can be used to treat chronic neurological diseases.
Conflict of interest statement
Figures
Similar articles
-
Ubiquitin-specific protease 14 regulates c-Jun N-terminal kinase signaling at the neuromuscular junction.Mol Neurodegener. 2015 Jan 10;10:3. doi: 10.1186/1750-1326-10-3. Mol Neurodegener. 2015. PMID: 25575639 Free PMC article.
-
An inhibitor of the proteasomal deubiquitinating enzyme USP14 induces tau elimination in cultured neurons.J Biol Chem. 2017 Nov 24;292(47):19209-19225. doi: 10.1074/jbc.M117.815126. Epub 2017 Sep 26. J Biol Chem. 2017. PMID: 28972160 Free PMC article.
-
Examination of genetic and pharmacological tools to study the proteasomal deubiquitinating enzyme ubiquitin-specific protease 14 in the nervous system.J Neurochem. 2021 Feb;156(3):309-323. doi: 10.1111/jnc.15180. Epub 2020 Sep 30. J Neurochem. 2021. PMID: 32901953 Free PMC article.
-
Neurotoxic mechanisms by which the USP14 inhibitor IU1 depletes ubiquitinated proteins and Tau in rat cerebral cortical neurons: Relevance to Alzheimer's disease.Biochim Biophys Acta Mol Basis Dis. 2017 Jun;1863(6):1157-1170. doi: 10.1016/j.bbadis.2017.03.017. Epub 2017 Apr 1. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28372990 Free PMC article.
-
USP14: Structure, Function, and Target Inhibition.Front Pharmacol. 2022 Jan 5;12:801328. doi: 10.3389/fphar.2021.801328. eCollection 2021. Front Pharmacol. 2022. PMID: 35069211 Free PMC article. Review.
Cited by
-
USP14 promotes pyroptosis of human annulus fibrosus cells derived from patients with intervertebral disc degeneration through deubiquitination of NLRP3.Acta Biochim Biophys Sin (Shanghai). 2022 Nov 25;54(11):1720 - 1730. doi: 10.3724/abbs.2022171. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36514221 Free PMC article.
-
Genetic background alters the severity and onset of neuromuscular disease caused by the loss of ubiquitin-specific protease 14 (usp14).PLoS One. 2013 Dec 16;8(12):e84042. doi: 10.1371/journal.pone.0084042. eCollection 2013. PLoS One. 2013. PMID: 24358326 Free PMC article.
-
Inhibition of 19S proteasome-associated deubiquitinases by metal-containing compounds.Oncoscience. 2015 May 31;2(5):457-66. doi: 10.18632/oncoscience.167. eCollection 2015. Oncoscience. 2015. PMID: 26097878 Free PMC article. Review.
-
Cellular and pathological functions of tau.Nat Rev Mol Cell Biol. 2024 Nov;25(11):845-864. doi: 10.1038/s41580-024-00753-9. Epub 2024 Jul 16. Nat Rev Mol Cell Biol. 2024. PMID: 39014245 Review.
-
An optimal ubiquitin-proteasome pathway in the nervous system: the role of deubiquitinating enzymes.Front Mol Neurosci. 2014 Aug 19;7:72. doi: 10.3389/fnmol.2014.00072. eCollection 2014. Front Mol Neurosci. 2014. PMID: 25191222 Free PMC article. Review.
References
-
- Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428. - PubMed
-
- Bingol B, Sheng M (2011) Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron 69: 22–32. - PubMed
-
- Burke RE (2004) Recent advances in research on Parkinson disease: synuclein and parkin. Neurologist 10: 75–81. - PubMed
-
- Wilkinson KD, Urban MK, Haas AL (1980) Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J Biol Chem 255: 7529–7532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

