TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals
- PMID: 23142541
- PMCID: PMC3935769
- DOI: 10.1016/j.yjmcc.2012.10.016
TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals
Abstract
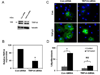
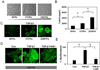
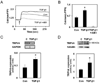
The phenotypic switch underlying the differentiation of cardiac fibroblasts into hypersecretory myofibroblasts is critical for cardiac remodeling following myocardial infarction. Myofibroblasts facilitate wound repair in the myocardium by secreting and organizing extracellular matrix (ECM) during the wound healing process. However, the molecular mechanisms involved in myofibroblast differentiation are not well known. TGF-β has been shown to promote differentiation and this, combined with the robust mechanical environment in the heart, lead us to hypothesize that the mechanotransduction and TGF-β signaling pathways play active roles in the differentiation of cardiac fibroblasts to myofibroblasts. Here, we show that the mechanosensitve ion channel TRPV4 is required for TGF-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. We found that the TRPV4-specific antagonist AB159908 and siRNA knockdown of TRPV4 significantly inhibited TGFβ1-induced differentiation as measured by incorporation of α-SMA into stress fibers. Further, we found that TGF-β1-induced myofibroblast differentiation was dependent on ECM stiffness, a response that was attenuated by TRPV4 blockade. Finally, TGF-β1 treated fibroblasts exhibited enhanced TRPV4 expression and TRPV4-mediated calcium influx compared to untreated controls. Taken together these results suggest for the first time that the mechanosensitive ion channel, TRPV4, regulates cardiac fibroblast differentiation to myofibroblasts by integrating signals from TGF-β1 and mechanical factors.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
A TRP to cardiac fibroblast differentiation.Channels (Austin). 2013 May-Jun;7(3):211-4. doi: 10.4161/chan.24328. Epub 2013 Mar 19. Channels (Austin). 2013. PMID: 23511028 Free PMC article.
Similar articles
-
TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation.Basic Res Cardiol. 2020 Jan 10;115(2):14. doi: 10.1007/s00395-020-0775-5. Basic Res Cardiol. 2020. PMID: 31925567 Free PMC article.
-
TRPV4 ion channel is a novel regulator of dermal myofibroblast differentiation.Am J Physiol Cell Physiol. 2017 May 1;312(5):C562-C572. doi: 10.1152/ajpcell.00187.2016. Epub 2017 Mar 1. Am J Physiol Cell Physiol. 2017. PMID: 28249987 Free PMC article.
-
A TRP to cardiac fibroblast differentiation.Channels (Austin). 2013 May-Jun;7(3):211-4. doi: 10.4161/chan.24328. Epub 2013 Mar 19. Channels (Austin). 2013. PMID: 23511028 Free PMC article.
-
TRPV4 Mechanotransduction in Fibrosis.Cells. 2021 Nov 6;10(11):3053. doi: 10.3390/cells10113053. Cells. 2021. PMID: 34831281 Free PMC article. Review.
-
Fibroblasts in post-infarction inflammation and cardiac repair.Biochim Biophys Acta. 2013 Apr;1833(4):945-53. doi: 10.1016/j.bbamcr.2012.08.023. Epub 2012 Sep 7. Biochim Biophys Acta. 2013. PMID: 22982064 Free PMC article. Review.
Cited by
-
The Multifaceted Functions of TRPV4 and Calcium Oscillations in Tissue Repair.Int J Mol Sci. 2024 Jan 18;25(2):1179. doi: 10.3390/ijms25021179. Int J Mol Sci. 2024. PMID: 38256251 Free PMC article. Review.
-
Transient Receptor Potential Vanilloid channel regulates fibroblast differentiation and airway remodeling by modulating redox signals through NADPH Oxidase 4.Sci Rep. 2020 Jun 17;10(1):9827. doi: 10.1038/s41598-020-66617-2. Sci Rep. 2020. PMID: 32555397 Free PMC article.
-
TRPM8-Rap1A Interaction Sites as Critical Determinants for Adhesion and Migration of Prostate and Other Epithelial Cancer Cells.Cancers (Basel). 2022 Apr 30;14(9):2261. doi: 10.3390/cancers14092261. Cancers (Basel). 2022. PMID: 35565390 Free PMC article.
-
TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation.Basic Res Cardiol. 2020 Jan 10;115(2):14. doi: 10.1007/s00395-020-0775-5. Basic Res Cardiol. 2020. PMID: 31925567 Free PMC article.
-
Transient receptor potential vanilloid 4 (TRPV4) activation by arachidonic acid requires protein kinase A-mediated phosphorylation.J Biol Chem. 2018 Apr 6;293(14):5307-5322. doi: 10.1074/jbc.M117.811075. Epub 2018 Feb 8. J Biol Chem. 2018. PMID: 29462784 Free PMC article.
References
-
- Flack EC, Lindsey ML, Squires CE, Kaplan BS, Stroud RE, Clark LL, et al. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J Mol Cell Cardiol. 2006;40(4):474–483. - PubMed
-
- Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, et al. Altered fibroblast function following myocardial infarction. J Mol Cell Cardiol. 2005;39(4):699–707. - PubMed
-
- Follonier Castella L, Gabbiani G, McCulloch CA, Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res. 2010;316(15):2390–23401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

