Methamphetamine reduces human influenza A virus replication
- PMID: 23139774
- PMCID: PMC3491060
- DOI: 10.1371/journal.pone.0048335
Methamphetamine reduces human influenza A virus replication
Abstract
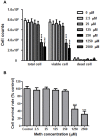
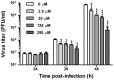
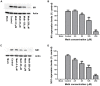
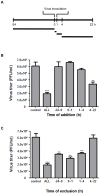
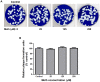
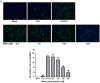
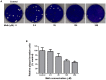
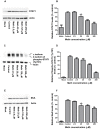
Methamphetamine (meth) is a highly addictive psychostimulant that is among the most widely abused illicit drugs, with an estimated over 35 million users in the world. Several lines of evidence suggest that chronic meth abuse is a major factor for increased risk of infections with human immunodeficiency virus and possibly other pathogens, due to its immunosuppressive property. Influenza A virus infections frequently cause epidemics and pandemics of respiratory diseases among human populations. However, little is known about whether meth has the ability to enhance influenza A virus replication, thus increasing severity of influenza illness in meth abusers. Herein, we investigated the effects of meth on influenza A virus replication in human lung epithelial A549 cells. The cells were exposed to meth and infected with human influenza A/WSN/33 (H1N1) virus. The viral progenies were titrated by plaque assays, and the expression of viral proteins and cellular proteins involved in interferon responses was examined by Western blotting and immunofluorescence staining. We report the first evidence that meth significantly reduces, rather than increases, virus propagation and the susceptibility to influenza infection in the human lung epithelial cell line, consistent with a decrease in viral protein synthesis. These effects were apparently not caused by meth's effects on enhancing virus-induced interferon responses in the host cells, reducing viral biological activities, or reducing cell viability. Our results suggest that meth might not be a great risk factor for influenza A virus infection among meth abusers. Although the underlying mechanism responsible for the action of meth on attenuating virus replication requires further investigation, these findings prompt the study to examine whether other structurally similar compounds could be used as anti-influenza agents.
Conflict of interest statement
Figures
Similar articles
-
A small molecule multi-kinase inhibitor reduces influenza A virus replication by restricting viral RNA synthesis.Antiviral Res. 2013 Oct;100(1):29-37. doi: 10.1016/j.antiviral.2013.07.010. Epub 2013 Jul 24. Antiviral Res. 2013. PMID: 23891991
-
14-Deoxy-11,12-dehydroandrographolide exerts anti-influenza A virus activity and inhibits replication of H5N1 virus by restraining nuclear export of viral ribonucleoprotein complexes.Antiviral Res. 2015 Jun;118:82-92. doi: 10.1016/j.antiviral.2015.03.008. Epub 2015 Mar 20. Antiviral Res. 2015. PMID: 25800824
-
Influenza Virus Infection, Interferon Response, Viral Counter-Response, and Apoptosis.Viruses. 2017 Aug 12;9(8):223. doi: 10.3390/v9080223. Viruses. 2017. PMID: 28805681 Free PMC article. Review.
-
Phosphatidylglycerol suppresses influenza A virus infection.Am J Respir Cell Mol Biol. 2012 Apr;46(4):479-87. doi: 10.1165/rcmb.2011-0194OC. Epub 2011 Nov 3. Am J Respir Cell Mol Biol. 2012. PMID: 22052877 Free PMC article.
-
Immunomodulatory Nonstructural Proteins of Influenza A Viruses.Trends Microbiol. 2018 Jul;26(7):624-636. doi: 10.1016/j.tim.2017.12.006. Epub 2018 Jan 17. Trends Microbiol. 2018. PMID: 29373257 Review.
Cited by
-
Peptide immunization against the C-terminal of alpha-synuclein reduces locomotor activity in mice overexpressing alpha-synuclein.PLoS One. 2023 Sep 21;18(9):e0291927. doi: 10.1371/journal.pone.0291927. eCollection 2023. PLoS One. 2023. PMID: 37733672 Free PMC article.
-
The potential of adoptive transfer of γ9δ2 T cells to enhance blinatumomab's antitumor activity against B-cell malignancy.Sci Rep. 2021 Jun 11;11(1):12398. doi: 10.1038/s41598-021-91784-1. Sci Rep. 2021. PMID: 34117317 Free PMC article.
-
Stimulants and the lung : review of literature.Clin Rev Allergy Immunol. 2014 Feb;46(1):82-100. doi: 10.1007/s12016-013-8376-9. Clin Rev Allergy Immunol. 2014. PMID: 23760760 Review.
-
Virtual Screen for Repurposing of Drugs for Candidate Influenza a M2 Ion-Channel Inhibitors.Front Cell Infect Microbiol. 2019 Mar 26;9:67. doi: 10.3389/fcimb.2019.00067. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30972303 Free PMC article.
-
Recombinant Adeno-Associated Virus-Mediated Expression of Methamphetamine Antibody Attenuates Methamphetamine-Induced Hyperactivity in Mice.Sci Rep. 2017 Apr 7;7:46301. doi: 10.1038/srep46301. Sci Rep. 2017. PMID: 28387350 Free PMC article.
References
-
- Colfax G, Shoptaw S (2005) The methamphetamine epidemic: implications for HIV prevention and treatment. Curr HIV/AIDS Rep 2: 194–199. - PubMed
-
- Ricaurte GA, Schuster CR, Seiden LS (1980) Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res 193: 153–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

