Identification of a tetrahydroquinoline analog as a pharmacological inhibitor of the cAMP-binding protein Epac
- PMID: 23139415
- PMCID: PMC3531735
- DOI: 10.1074/jbc.M112.422956
Identification of a tetrahydroquinoline analog as a pharmacological inhibitor of the cAMP-binding protein Epac
Abstract
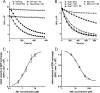
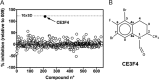
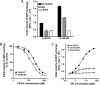
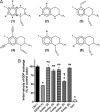
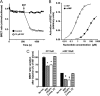
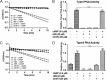
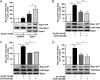
The cAMP-binding protein Epac is a therapeutic target for the treatment of various diseases such as cardiac hypertrophy and tumor invasion. This points out the importance to develop Epac inhibitors to better understand the involvement of these cAMP sensors in physiology and pathophysiology. Here, we have developed a functional fluorescence-based high-throughput assay with a Z' value around 0.7 for screening Epac-specific antagonists. We identified an Epac1 inhibitor compound named CE3F4 that blocked Epac1 guanine nucleotide exchange activity toward its effector Rap1 both in cell-free systems and in intact cells. CE3F4 is a tetrahydroquinoline analog that fails to influence protein kinase A holoenzyme activity. CE3F4 inhibited neither the interaction of Rap1 with Epac1 nor directly the GDP exchange on Rap1. The kinetics of inhibition by CE3F4 indicated that this compound did not compete for binding of agonists to Epac1 and suggested an uncompetitive inhibition mechanism with respect to Epac1 agonists. A structure-activity study showed that the formyl group on position 1 and the bromine atom on position 5 of the tetrahydroquinoline skeleton were important for CE3F4 to exert its inhibitory activity. Finally, CE3F4 inhibited Rap1 activation in living cultured cells, following Epac activation by either 8-(4-chlorophenylthio)-2'-O-methyl-cAMP, an Epac-selective agonist, or isoprenaline, a non-selective β-adrenergic receptor agonist. Our study shows that CE3F4 and related compounds may serve as a basis for the development of new therapeutic drugs.
Figures

Similar articles
-
The (R)-enantiomer of CE3F4 is a preferential inhibitor of human exchange protein directly activated by cyclic AMP isoform 1 (Epac1).Biochem Biophys Res Commun. 2013 Oct 25;440(3):443-8. doi: 10.1016/j.bbrc.2013.09.107. Epub 2013 Oct 4. Biochem Biophys Res Commun. 2013. PMID: 24099776
-
Identification and validation of modulators of exchange protein activated by cAMP (Epac) activity: structure-function implications for Epac activation and inhibition.J Biol Chem. 2014 Mar 21;289(12):8217-30. doi: 10.1074/jbc.M114.548636. Epub 2014 Feb 4. J Biol Chem. 2014. PMID: 24497631 Free PMC article.
-
Activation of G protein-coupled estrogen receptor 1 induces coronary artery relaxation via Epac/Rap1-mediated inhibition of RhoA/Rho kinase pathway in parallel with PKA.PLoS One. 2017 Mar 9;12(3):e0173085. doi: 10.1371/journal.pone.0173085. eCollection 2017. PLoS One. 2017. PMID: 28278256 Free PMC article.
-
Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology.Pflugers Arch. 2010 Mar;459(4):535-46. doi: 10.1007/s00424-009-0747-y. Epub 2009 Oct 25. Pflugers Arch. 2010. PMID: 19855995 Review.
-
The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome and Pathophysiology.Cells. 2019 Nov 29;8(12):1543. doi: 10.3390/cells8121543. Cells. 2019. PMID: 31795450 Free PMC article. Review.
Cited by
-
cAMP signaling through protein kinase A and Epac2 induces substance P release in the rat spinal cord.Neuropharmacology. 2021 May 15;189:108533. doi: 10.1016/j.neuropharm.2021.108533. Epub 2021 Mar 17. Neuropharmacology. 2021. PMID: 33744339 Free PMC article.
-
Functionalized N,N-Diphenylamines as Potent and Selective EPAC2 Inhibitors.ACS Med Chem Lett. 2016 Mar 28;7(5):460-4. doi: 10.1021/acsmedchemlett.5b00477. eCollection 2016 May 12. ACS Med Chem Lett. 2016. PMID: 27190593 Free PMC article.
-
EPAC1 and EPAC2 promote nociceptor hyperactivity associated with chronic pain after spinal cord injury.Neurobiol Pain. 2019 Dec 4;7:100040. doi: 10.1016/j.ynpai.2019.100040. eCollection 2020 Jan-Jul. Neurobiol Pain. 2019. PMID: 31890991 Free PMC article.
-
A-kinase anchoring proteins: cAMP compartmentalization in neurodegenerative and obstructive pulmonary diseases.Br J Pharmacol. 2014 Dec;171(24):5603-23. doi: 10.1111/bph.12882. Br J Pharmacol. 2014. PMID: 25132049 Free PMC article. Review.
-
Ca2+ influx through L-type Ca2+ channels and Ca2+-induced Ca2+ release regulate cAMP accumulation and Epac1-dependent ERK 1/2 activation in INS-1 cells.Mol Cell Endocrinol. 2016 Jan 5;419:60-71. doi: 10.1016/j.mce.2015.09.034. Epub 2015 Oct 3. Mol Cell Endocrinol. 2016. PMID: 26435461 Free PMC article.
References
-
- Breckler M., Berthouze M., Laurent A. C., Crozatier B., Morel E., Lezoualc'h F. (2011) Rap-linked cAMP signaling Epac proteins. Compartmentation, functioning and disease implications. Cell. Signal. 23, 1257–1266 - PubMed
-
- Métrich M., Berthouze M., Morel E., Crozatier B., Gomez A. M., Lezoualc'h F. (2010) Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflugers Arch. 459, 535–546 - PubMed
-
- Kawasaki H., Springett G. M., Mochizuki N., Toki S., Nakaya M., Matsuda M., Housman D. E., Graybiel A. M. (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–2279 - PubMed
-
- de Rooij J., Zwartkruis F. J., Verheijen M. H., Cool R. H., Nijman S. M., Wittinghofer A., Bos J. L. (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–477 - PubMed
-
- Gloerich M., Bos J. L. (2010) Epac. Defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 50, 355–375 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

