Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models
- PMID: 23138870
- PMCID: PMC3810211
- DOI: 10.1038/cgt.2012.75
Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models
Abstract
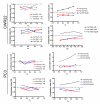
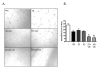
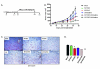
Oncolytic herpes simplex virus (oHSV)-1-based vectors selectively replicate in tumor cells causing direct killing, that is, oncolysis, while sparing normal cells. The oHSVs are promising anticancer agents, but their efficacy, when used as single agents, leaves room for improvement. We hypothesized that combining the direct oncolytic and antiangiogenic activities of the interleukin (IL)-12-secreting NV1042 oHSV with microtubule disrupting agents (MDAs) would be an effective means to enhance antitumor efficacy. Vinblastine (VB) was identified among several MDAs screened, which displayed consistent and potent cytotoxic killing of both prostate cancer and endothelial cell lines. In matrigel tube-forming assays, VB was found to be highly effective at inhibiting tube formation of human umbilical vein endothelial cells. The combination of VB with NV1023 (the parental virus lacking IL-12) or NV1042 showed additive or synergistic activity against prostate cancer cell lines, and was not due to increased oHSV replication by VB. In athymic mice bearing CWR22 prostate tumors, VB in combination with NV1042 was superior to the combination of VB plus NV1023 in reducing tumor burden, appeared to be nontoxic and resulted in a statistically significant diminution in the number of CD31(+) cells as compared with other treatment groups. In human organotypic cultures using surgical samples from radical prostatectomies, both NV1023 and NV1042 were localized specifically to the epithelial cells of prostatic glands but not to the surrounding stroma. These data highlight the therapeutic advantage of combining the dual-acting antitumor and antiangiogenic activities of oHSVs and MDAs.
Figures
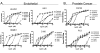
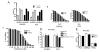

Similar articles
-
Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers.Cancer Gene Ther. 2006 Mar;13(3):253-65. doi: 10.1038/sj.cgt.7700900. Cancer Gene Ther. 2006. PMID: 16179929
-
An oncolytic herpes simplex virus vector, G47Δ, synergizes with paclitaxel in the treatment of breast cancer.Oncol Rep. 2013 Jun;29(6):2355-61. doi: 10.3892/or.2013.2359. Epub 2013 Mar 22. Oncol Rep. 2013. PMID: 23525624
-
Interleukin 12 secretion enhances antitumor efficacy of oncolytic herpes simplex viral therapy for colorectal cancer.Ann Surg. 2001 Jun;233(6):819-26. doi: 10.1097/00000658-200106000-00012. Ann Surg. 2001. PMID: 11371740 Free PMC article.
-
Herpes Simplex Virus Oncolytic Immunovirotherapy: The Blossoming Branch of Multimodal Therapy.Int J Mol Sci. 2020 Nov 5;21(21):8310. doi: 10.3390/ijms21218310. Int J Mol Sci. 2020. PMID: 33167582 Free PMC article. Review.
-
Oncolytic Herpes Simplex Virus-Based Therapies for Cancer.Cells. 2021 Jun 18;10(6):1541. doi: 10.3390/cells10061541. Cells. 2021. PMID: 34207386 Free PMC article. Review.
Cited by
-
Oncolytic herpes simplex virus immunovirotherapy in combination with immune checkpoint blockade to treat glioblastoma.Immunotherapy. 2018 Jul;10(9):779-786. doi: 10.2217/imt-2018-0009. Immunotherapy. 2018. PMID: 30008259 Free PMC article.
-
A Double-Edged Sword Role of Cytokines in Prostate Cancer Immunotherapy.Front Oncol. 2021 Nov 16;11:688489. doi: 10.3389/fonc.2021.688489. eCollection 2021. Front Oncol. 2021. PMID: 34868907 Free PMC article. Review.
-
New insights into IL-12-mediated tumor suppression.Cell Death Differ. 2015 Feb;22(2):237-46. doi: 10.1038/cdd.2014.134. Epub 2014 Sep 5. Cell Death Differ. 2015. PMID: 25190142 Free PMC article. Review.
-
Interleukin-12 Delivery Strategies and Advances in Tumor Immunotherapy.Curr Issues Mol Biol. 2024 Oct 16;46(10):11548-11579. doi: 10.3390/cimb46100686. Curr Issues Mol Biol. 2024. PMID: 39451566 Free PMC article. Review.
-
Oncolyic Virotherapy for Prostate Cancer: Lighting a Fire in Winter.Int J Mol Sci. 2022 Oct 21;23(20):12647. doi: 10.3390/ijms232012647. Int J Mol Sci. 2022. PMID: 36293504 Free PMC article. Review.
References
-
- Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Science. 1991;252:854–856. - PubMed
-
- Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:939–943. - PubMed
-
- Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–978. - PubMed
-
- Kuruppu D, Tanabe KK. Viral oncolysis by herpes simplex virus and other viruses. Cancer BiolTher. 2005;4:524–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

