Co-transcriptional nuclear actin dynamics
- PMID: 23138849
- PMCID: PMC3585027
- DOI: 10.4161/nucl.22798
Co-transcriptional nuclear actin dynamics
Abstract
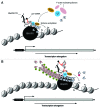
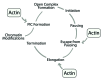
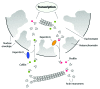
Actin is a key player for nuclear structure and function regulating both chromosome organization and gene activity. In the cell nucleus actin interacts with many different proteins. Among these proteins several studies have identified classical nuclear factors involved in chromatin structure and function, transcription and RNA processing as well as proteins that are normally involved in controlling the actin cytoskeleton. These discoveries have raised the possibility that nuclear actin performs its multi task activities through tight interactions with different sets of proteins. This high degree of promiscuity in the spectrum of protein-to-protein interactions correlates well with the conformational plasticity of actin and the ability to undergo regulated changes in its polymerization states. Several of the factors involved in controlling head-to-tail actin polymerization have been shown to be in the nucleus where they seem to regulate gene activity. By focusing on the multiple tasks performed by actin and actin-binding proteins, possible models of how actin dynamics controls the different phases of the RNA polymerase II transcription cycle are being identified.
Keywords: Nuclear actin; RNA polymerase; actin polymerization; nuclear structure and function; transcription.
Figures


Similar articles
-
Rho, nuclear actin, and actin-binding proteins in the regulation of transcription and gene expression.Small GTPases. 2014;5:e27539. doi: 10.4161/sgtp.27539. Epub 2014 Mar 6. Small GTPases. 2014. PMID: 24603113 Free PMC article. Review.
-
Persistent nuclear actin filaments inhibit transcription by RNA polymerase II.J Cell Sci. 2016 Sep 15;129(18):3412-25. doi: 10.1242/jcs.195867. Epub 2016 Aug 2. J Cell Sci. 2016. PMID: 27505898 Free PMC article.
-
Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners.Nat Cell Biol. 2006 Jul;8(7):756-63. doi: 10.1038/ncb1433. Epub 2006 Jun 11. Nat Cell Biol. 2006. PMID: 16767080
-
Nuclear actin and actin-related proteins in chromatin dynamics.Curr Opin Cell Biol. 2007 Jun;19(3):326-30. doi: 10.1016/j.ceb.2007.04.009. Epub 2007 Apr 27. Curr Opin Cell Biol. 2007. PMID: 17467255 Review.
-
Searching for a function for nuclear actin.Trends Cell Biol. 2000 Mar;10(3):92-7. doi: 10.1016/s0962-8924(99)01713-4. Trends Cell Biol. 2000. PMID: 10675902 Review.
Cited by
-
Nuclear myosin 1 contributes to a chromatin landscape compatible with RNA polymerase II transcription activation.BMC Biol. 2015 Jun 5;13:35. doi: 10.1186/s12915-015-0147-z. BMC Biol. 2015. PMID: 26044184 Free PMC article.
-
β-actin contributes to open chromatin for activation of the adipogenic pioneer factor CEBPA during transcriptional reprograming.Mol Biol Cell. 2020 Nov 1;31(23):2511-2521. doi: 10.1091/mbc.E19-11-0628. Epub 2020 Sep 2. Mol Biol Cell. 2020. PMID: 32877276 Free PMC article.
-
Transcriptional regulation and nuclear reprogramming: roles of nuclear actin and actin-binding proteins.Cell Mol Life Sci. 2013 Sep;70(18):3289-302. doi: 10.1007/s00018-012-1235-7. Epub 2012 Dec 29. Cell Mol Life Sci. 2013. PMID: 23275942 Free PMC article. Review.
-
Nuclear actin regulates inducible transcription by enhancing RNA polymerase II clustering.Sci Adv. 2020 Apr 15;6(16):eaay6515. doi: 10.1126/sciadv.aay6515. eCollection 2020 Apr. Sci Adv. 2020. PMID: 32494599 Free PMC article.
-
To be or not to be assembled: progressing into nuclear actin filaments.Nat Rev Mol Cell Biol. 2013 Nov;14(11):693-7. doi: 10.1038/nrm3681. Epub 2013 Oct 3. Nat Rev Mol Cell Biol. 2013. PMID: 24088744 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
