Engagement of the ATR-dependent DNA damage response at the human papillomavirus 18 replication centers during the initial amplification
- PMID: 23135710
- PMCID: PMC3554080
- DOI: 10.1128/JVI.01943-12
Engagement of the ATR-dependent DNA damage response at the human papillomavirus 18 replication centers during the initial amplification
Abstract
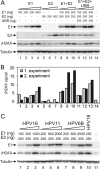
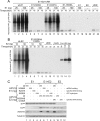
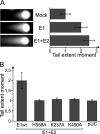
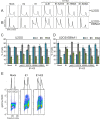
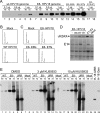
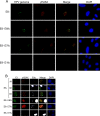
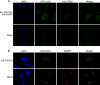
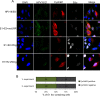
We have previously demonstrated that the human papillomavirus (HPV) genome replicates effectively in U2OS cells after transfection using electroporation. The transient extrachromosomal replication, stable maintenance, and late amplification of the viral genome could be studied for high- and low-risk mucosal and cutaneous papillomaviruses. Recent findings indicate that the cellular DNA damage response (DDR) is activated during the HPV life cycle and that the viral replication protein E1 might play a role in this process. We used a U2OS cell-based system to study E1-dependent DDR activation and the involvement of these pathways in viral transient replication. We demonstrated that the E1 protein could cause double-strand DNA breaks in the host genome by directly interacting with DNA. This activity leads to the induction of an ATM-dependent signaling cascade and cell cycle arrest in the S and G(2) phases. However, the transient replication of HPV genomes in U2OS cells induces the ATR-dependent pathway, as shown by the accumulation of γH2AX, ATR-interacting protein (ATRIP), and topoisomerase IIβ-binding protein 1 (TopBP1) in viral replication centers. Viral oncogenes do not play a role in this activation, which is induced only through DNA replication or by replication proteins E1 and E2. The ATR pathway in viral replication centers is likely activated through DNA replication stress and might play an important role in engaging cellular DNA repair/recombination machinery for effective replication of the viral genome upon active amplification.
Figures

Similar articles
-
Human Papillomaviruses Preferentially Recruit DNA Repair Factors to Viral Genomes for Rapid Repair and Amplification.mBio. 2018 Feb 13;9(1):e00064-18. doi: 10.1128/mBio.00064-18. mBio. 2018. PMID: 29440569 Free PMC article.
-
STAT-5 Regulates Transcription of the Topoisomerase IIβ-Binding Protein 1 (TopBP1) Gene To Activate the ATR Pathway and Promote Human Papillomavirus Replication.mBio. 2015 Dec 22;6(6):e02006-15. doi: 10.1128/mBio.02006-15. mBio. 2015. PMID: 26695634 Free PMC article.
-
Nuclear accumulation of the papillomavirus E1 helicase blocks S-phase progression and triggers an ATM-dependent DNA damage response.J Virol. 2011 Sep;85(17):8996-9012. doi: 10.1128/JVI.00542-11. Epub 2011 Jul 6. J Virol. 2011. PMID: 21734051 Free PMC article.
-
Why Human Papillomaviruses Activate the DNA Damage Response (DDR) and How Cellular and Viral Replication Persists in the Presence of DDR Signaling.Viruses. 2017 Sep 21;9(10):268. doi: 10.3390/v9100268. Viruses. 2017. PMID: 28934154 Free PMC article. Review.
-
Modulation of the DNA damage response during the life cycle of human papillomaviruses.Virus Res. 2017 Mar 2;231:41-49. doi: 10.1016/j.virusres.2016.11.006. Epub 2016 Nov 9. Virus Res. 2017. PMID: 27836727 Free PMC article. Review.
Cited by
-
High-risk human papillomavirus oncogenes disrupt the Fanconi anemia DNA repair pathway by impairing localization and de-ubiquitination of FancD2.PLoS Pathog. 2019 Feb 28;15(2):e1007442. doi: 10.1371/journal.ppat.1007442. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30818369 Free PMC article.
-
Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability.Genome Res. 2014 Feb;24(2):185-99. doi: 10.1101/gr.164806.113. Epub 2013 Nov 7. Genome Res. 2014. PMID: 24201445 Free PMC article.
-
The DNA damage response induced by infection with human cytomegalovirus and other viruses.Viruses. 2014 May 23;6(5):2155-85. doi: 10.3390/v6052155. Viruses. 2014. PMID: 24859341 Free PMC article. Review.
-
KDM6A addiction of cervical carcinoma cell lines is triggered by E7 and mediated by p21CIP1 suppression of replication stress.PLoS Pathog. 2017 Oct 2;13(10):e1006661. doi: 10.1371/journal.ppat.1006661. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 28968467 Free PMC article.
-
Regulation of host factor γ-H2AX level and location by enterovirus A71 for viral replication.Virulence. 2022 Dec;13(1):241-257. doi: 10.1080/21505594.2022.2028482. Virulence. 2022. PMID: 35067196 Free PMC article.
References
-
- Howley PM, Lowy DR. 2001. Papillomaviruses and their replication, p 2197–2230 In Knipe DM, Howley PM. (ed), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA
-
- Broker TR, Jin G, Croom-Rivers A, Bragg SM, Richardson M, Chow LT, Vermund SH, Alvarez RD, Pappas PG, Squires KE, Hoesley CJ. 2001. Viral latency—the papillomavirus model. Dev. Biol. 106:443–451 - PubMed
-
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338:423–428 - PubMed
-
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. 2004. Classification of papillomaviruses. Virology 324:17–27 - PubMed
-
- zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2:342–350 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

