Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction
- PMID: 23135401
- PMCID: PMC3518760
- DOI: 10.1038/nature11590
Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction
Abstract
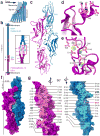
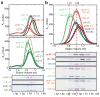
Hearing and balance use hair cells in the inner ear to transform mechanical stimuli into electrical signals. Mechanical force from sound waves or head movements is conveyed to hair-cell transduction channels by tip links, fine filaments formed by two atypical cadherins known as protocadherin 15 and cadherin 23 (refs 4, 5). These two proteins are involved in inherited deafness and feature long extracellular domains that interact tip-to-tip in a Ca(2+)-dependent manner. However, the molecular architecture of this complex is unknown. Here we combine crystallography, molecular dynamics simulations and binding experiments to characterize the protocadherin 15-cadherin 23 bond. We find a unique cadherin interaction mechanism, in which the two most amino-terminal cadherin repeats (extracellular cadherin repeats 1 and 2) of each protein interact to form an overlapped, antiparallel heterodimer. Simulations predict that this tip-link bond is mechanically strong enough to resist forces in hair cells. In addition, the complex is shown to become unstable in response to Ca(2+) removal owing to increased flexure of Ca(2+)-free cadherin repeats. Finally, we use structures and biochemical measurements to study the molecular mechanisms by which deafness mutations disrupt tip-link function. Overall, our results shed light on the molecular mechanics of hair-cell sensory transduction and on new interaction mechanisms for cadherins, a large protein family implicated in tissue and organ morphogenesis, neural connectivity and cancer.
Figures


Similar articles
-
Tuning Inner-Ear Tip-Link Affinity Through Alternatively Spliced Variants of Protocadherin-15.Biochemistry. 2018 Mar 20;57(11):1702-1710. doi: 10.1021/acs.biochem.7b01075. Epub 2018 Mar 6. Biochemistry. 2018. PMID: 29443515 Free PMC article.
-
Structural determinants of cadherin-23 function in hearing and deafness.Neuron. 2010 Apr 15;66(1):85-100. doi: 10.1016/j.neuron.2010.03.028. Neuron. 2010. PMID: 20399731 Free PMC article.
-
Noddy, a mouse harboring a missense mutation in protocadherin-15, reveals the impact of disrupting a critical interaction site between tip-link cadherins in inner ear hair cells.J Neurosci. 2013 Mar 6;33(10):4395-404. doi: 10.1523/JNEUROSCI.4514-12.2013. J Neurosci. 2013. PMID: 23467356 Free PMC article.
-
Tip links in hair cells: molecular composition and role in hearing loss.Curr Opin Otolaryngol Head Neck Surg. 2009 Oct;17(5):388-93. doi: 10.1097/MOO.0b013e3283303472. Curr Opin Otolaryngol Head Neck Surg. 2009. PMID: 19633555 Free PMC article. Review.
-
Cadherins and mechanotransduction by hair cells.Curr Opin Cell Biol. 2008 Oct;20(5):557-66. doi: 10.1016/j.ceb.2008.06.004. Epub 2008 Jul 30. Curr Opin Cell Biol. 2008. PMID: 18619539 Free PMC article. Review.
Cited by
-
Structural determinants of adhesion by Protocadherin-19 and implications for its role in epilepsy.Elife. 2016 Oct 26;5:e18529. doi: 10.7554/eLife.18529. Elife. 2016. PMID: 27787195 Free PMC article.
-
Diversity of the Genes Implicated in Algerian Patients Affected by Usher Syndrome.PLoS One. 2016 Sep 1;11(9):e0161893. doi: 10.1371/journal.pone.0161893. eCollection 2016. PLoS One. 2016. PMID: 27583663 Free PMC article.
-
Structural basis of the strong cell-cell junction formed by cadherin-23.FEBS J. 2019 Nov 15;287(11):2328-47. doi: 10.1111/febs.15141. Online ahead of print. FEBS J. 2019. PMID: 31729176 Free PMC article.
-
Function and Dysfunction of TMC Channels in Inner Ear Hair Cells.Cold Spring Harb Perspect Med. 2019 Oct 1;9(10):a033506. doi: 10.1101/cshperspect.a033506. Cold Spring Harb Perspect Med. 2019. PMID: 30291150 Free PMC article. Review.
-
Interferon regulatory factor-7 is required for hair cell development during zebrafish embryogenesis.Dev Neurobiol. 2022 Jan;82(1):88-97. doi: 10.1002/dneu.22860. Epub 2021 Dec 22. Dev Neurobiol. 2022. PMID: 34779143 Free PMC article.
References
-
- Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of corti, and their possible relation to sensory transduction. Hear Res. 1984;15:103–112. - PubMed
-
- Assad JA, Shepherd G, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. - PubMed
-
- Kazmierczak P, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

