Positron emission tomography (PET) imaging with (18)F-based radiotracers
- PMID: 23133802
- PMCID: PMC3478111
Positron emission tomography (PET) imaging with (18)F-based radiotracers
Abstract
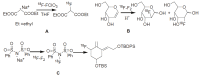
Positron Emission Tomography (PET) is a nuclear medicine imaging technique that is widely used in early detection and treatment follow up of many diseases, including cancer. This modality requires positron-emitting isotope labeled biomolecules, which are synthesized prior to perform imaging studies. Fluorine-18 is one of the several isotopes of fluorine that is routinely used in radiolabeling of biomolecules for PET; because of its positron emitting property and favorable half-life of 109.8 min. The biologically active molecule most commonly used for PET is 2-deoxy-2-(18)F-fluoro-β-D-glucose ((18)F-FDG), an analogue of glucose, for early detection of tumors. The concentrations of tracer accumulation (PET image) demonstrate the metabolic activity of tissues in terms of regional glucose metabolism and accumulation. Other tracers are also used in PET to image the tissue concentration. In this review, information on fluorination and radiofluorination reactions, radiofluorinating agents, and radiolabeling of various compounds and their application in PET imaging is presented.
Keywords: Fluorine-18; PET radiopharmaceuticals; positron emission tomography (PET).
Figures
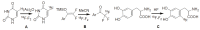
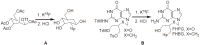
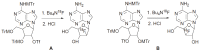
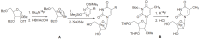
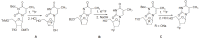
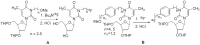
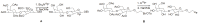
Similar articles
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Abass Alavi: A giant in Nuclear Medicine turns 80 and is still going strong!Hell J Nucl Med. 2018 Jan-Apr;21(1):85-87. doi: 10.1967/s002449910713. Epub 2018 Mar 20. Hell J Nucl Med. 2018. PMID: 29550853
-
Fluorine-18: Radiochemistry and Target-Specific PET Molecular Probes Design.Front Chem. 2022 Jun 29;10:884517. doi: 10.3389/fchem.2022.884517. eCollection 2022. Front Chem. 2022. PMID: 35844642 Free PMC article. Review.
-
[18F]Tosyl fluoride as a versatile [18F]fluoride source for the preparation of 18F-labeled radiopharmaceuticals.Sci Rep. 2023 Feb 23;13(1):3182. doi: 10.1038/s41598-023-30200-2. Sci Rep. 2023. PMID: 36823435 Free PMC article.
-
Development of (18)F-labeled radiotracers for neuroreceptor imaging with positron emission tomography.Neurosci Bull. 2014 Oct;30(5):777-811. doi: 10.1007/s12264-014-1460-6. Epub 2014 Aug 29. Neurosci Bull. 2014. PMID: 25172118 Free PMC article. Review.
Cited by
-
Positron emission tomography imaging of tumor angiogenesis with a 66Ga-labeled monoclonal antibody.Mol Pharm. 2012 May 7;9(5):1441-8. doi: 10.1021/mp300019c. Epub 2012 Apr 21. Mol Pharm. 2012. PMID: 22519890 Free PMC article.
-
ImmunoPET and near-infrared fluorescence imaging of CD105 expression using a monoclonal antibody dual-labeled with (89)Zr and IRDye 800CW.Am J Transl Res. 2012;4(3):333-46. Epub 2012 Jul 27. Am J Transl Res. 2012. PMID: 22937210 Free PMC article.
-
Aβ-Targeting Bifunctional Chelators (BFCs) for Potential Therapeutic and PET Imaging Applications.Int J Mol Sci. 2022 Dec 23;24(1):236. doi: 10.3390/ijms24010236. Int J Mol Sci. 2022. PMID: 36613679 Free PMC article. Review.
-
Recent advances in intermolecular 1,2-difunctionalization of alkenes involving trifluoromethylthiolation.RSC Adv. 2021 Jul 13;11(40):24474-24486. doi: 10.1039/d1ra02606b. eCollection 2021 Jul 13. RSC Adv. 2021. PMID: 35481061 Free PMC article. Review.
-
Imaging-guided delivery of RNAi for anticancer treatment.Adv Drug Deliv Rev. 2016 Sep 1;104:44-60. doi: 10.1016/j.addr.2016.01.008. Epub 2016 Jan 22. Adv Drug Deliv Rev. 2016. PMID: 26805788 Free PMC article. Review.
References
-
- Fluorine Chemistry. http://www.washington.edu/research/pathbreakers/1948b.html.
-
- O'Hagan D. "Understanding organofluorine chemistry. An introduction to the C-F bond". Chem Soc Rev. 2008;37:308–319. - PubMed
-
- Thayer AM. Fabulous Fluorine. Chemical and Engineering News. 2006;84:15–24.
-
- Suda Y, Shimidzu K, Sumi M, Oku N, Kusumoto S, Nadai T, Yamashita S. The synthesis and in vitro and in vivo stability of 5-fluorouracil prodrugs which possess serum albumin binding potency. Biol Pharmaceutical Bulletin. 1993;16:876–878. - PubMed
-
- Saleem A, Aboagye EO, Matthews JC, Price PM. Plasma pharmacokinetic evaluation of cytotoxic agents radiolabelled with positron emitting radioisotopes. Cancer Chemother Pharmacol. 2008;61:865–873. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
