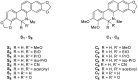
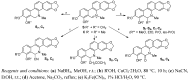
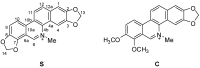In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi
- PMID: 23124471
- PMCID: PMC6268840
- DOI: 10.3390/molecules171113026
In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi
Abstract
In order to understand the antifungal activity of some derivatives of sanguinarine (S) and chelerythrine (C) and their structure-activity relationships, sixteen derivatives of S and C were prepared and evaluated for in vitro antifungal activity against seven phytopathogenic fungi by the mycelial growth rate method. The results showed that S, C and their 6-alkoxy dihydro derivatives S₁-S₄, C₁-C₄ and 6-cyanodihydro derivatives S₅, C₅ showed significant antifungal activity at 100 µg/mL against all the tested fungi. For most tested fungi, the median effective concentrations of S, S₁, C and C₁ were in a range of 14-50 µg/mL. The structure-activity relationship showed that the C=N+ moiety was the determinant for the antifungal activity of S and C. S₁-S₅ and C₁-C₅ could be considered as the precursors of S and C, respectively. Thus, the present results strongly suggested that S and C or their derivatives S₁-S₅ and C₁-C₅ should be considered as good lead compounds or model molecules to develop new anti-phytopathogenic fungal agents. can't login to work station for 2hrs--took 2 hrs vacation
Figures
Similar articles
-
2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as novel antifungal lead compounds: biological evaluation and structure-activity relationships.Molecules. 2013 Aug 29;18(9):10413-24. doi: 10.3390/molecules180910413. Molecules. 2013. PMID: 23994968 Free PMC article.
-
Inhibitory activity of dihydrosanguinarine and dihydrochelerythrine against phytopathogenic fungi.Nat Prod Res. 2011 Jul;25(11):1082-9. doi: 10.1080/14786419.2010.487187. Nat Prod Res. 2011. PMID: 21500094
-
Anti-microbial and anti-biofilm activities of combined chelerythrine-sanguinarine and mode of action against Candida albicans and Cryptococcus neoformans in vitro.Colloids Surf B Biointerfaces. 2020 Jul;191:111003. doi: 10.1016/j.colsurfb.2020.111003. Epub 2020 Apr 1. Colloids Surf B Biointerfaces. 2020. PMID: 32276211
-
Effects of sanguinarine and chelerythrine on the cell cycle and apoptosis.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006 Jul;150(1):5-12. doi: 10.5507/bp.2006.001. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006. PMID: 16936897 Review.
-
Azasteroids as antifungals.Steroids. 2003 Sep;68(7-8):587-94. doi: 10.1016/s0039-128x(03)00080-1. Steroids. 2003. PMID: 12957663 Review.
Cited by
-
Sanguinarine Inhibits Mono- and Dual-Species Biofilm Formation by Candida albicans and Staphylococcus aureus and Induces Mature Hypha Transition of C. albicans.Pharmaceuticals (Basel). 2020 Jan 13;13(1):13. doi: 10.3390/ph13010013. Pharmaceuticals (Basel). 2020. Retraction in: Pharmaceuticals (Basel). 2021 Feb 26;14(3):193. doi: 10.3390/ph14030193. PMID: 31941090 Free PMC article. Retracted.
-
Efficacy of chelerythrine against dual-species biofilms of Staphylococcus aureus and Staphylococcus lugdunensis.3 Biotech. 2020 Oct;10(10):427. doi: 10.1007/s13205-020-02401-3. Epub 2020 Sep 11. 3 Biotech. 2020. PMID: 32968612 Free PMC article.
-
Chelerythrine induces apoptosis via ROS-mediated endoplasmic reticulum stress and STAT3 pathways in human renal cell carcinoma.J Cell Mol Med. 2020 Jan;24(1):50-60. doi: 10.1111/jcmm.14295. Epub 2019 Sep 30. J Cell Mol Med. 2020. PMID: 31568643 Free PMC article.
-
Natural Protein Kinase Inhibitors, Staurosporine, and Chelerythrine Suppress Wheat Blast Disease Caused by Magnaporthe oryzae Triticum.Microorganisms. 2022 Jun 9;10(6):1186. doi: 10.3390/microorganisms10061186. Microorganisms. 2022. PMID: 35744705 Free PMC article.
-
Synthesis and Antileukemia Activity Evaluation of Benzophenanthridine Alkaloid Derivatives.Molecules. 2022 Jun 19;27(12):3934. doi: 10.3390/molecules27123934. Molecules. 2022. PMID: 35745057 Free PMC article.
References
-
- Wedge D.E., Camper N.D. Biologically Active Natural Products. In: Cutler H.G., Cutler S.J., editors. Agrochemicals and Pharmaceuticals. CRC Press; Boca Raton, FL, USA: 2000. pp. 1–15.
-
- Krane B.D., Fagbule M.O., Shamma M., Gözler B. The benzophenanthridine alkaloids. J. Nat. Prod. 1984;47:1–43. doi: 10.1021/np50031a001. - DOI
-
- Šimánek V. Benzophenanthridine Alkaloids. In: Brossi A., editor. The Alkaloids. Volume 26. Academic Press; New York, NY, USA: 1985. pp. 185–240.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous